
Using Materials (chemistry only)
Using Resources
Corrosion
Corrosion is the destruction of materials by chemical reactions with oxygen in the air. When metals corrode they are oxidised, to form metal oxides. Metals may form a thin layer of tarnish when they oxidise. This layer stops oxygen reaching the metal, preventing further oxidation.
Rusting is a specific example of corrosion, and requires both air and water for iron (or steel) to rust. There are three ways to prevent iron from rusting:
- exclusion of oxygen (by either using an atmosphere of nitrogen or argon)
- exclusion of water (by storing with a desiccant)
- sacrificial protection (when a more reactive metal than iron is attached to the object to prevent iron from rusting)
-
galvanising is a specific example of scarifical protection where a thin layer of zinc coats iron or steel, and can be carried out either be electrolysis or dipping the object in molten zinc
Corrosion can also be prevented by using a physical barrier (e.g. applying a coating such as greasing, painting, or plastic).
Electroplating
Electrolysis can be used to put a thin layer of a metal on an object that you don't want to corrode. An example is steel cutlery being electroplated with silver. It requires:
- the cathode to be the object you want electroplated (e.g. steel cutlery)
- the anode to be the metal you are plating the object with (e.g. silver)
- the electrolyte to contain ions of the metal found at the anode (e.g. silver nitrate solution)
Electroplating is used to improve the corrosion resistance of metal objects, but also is used to improve appearance (e.g. gold-plating jewellery).
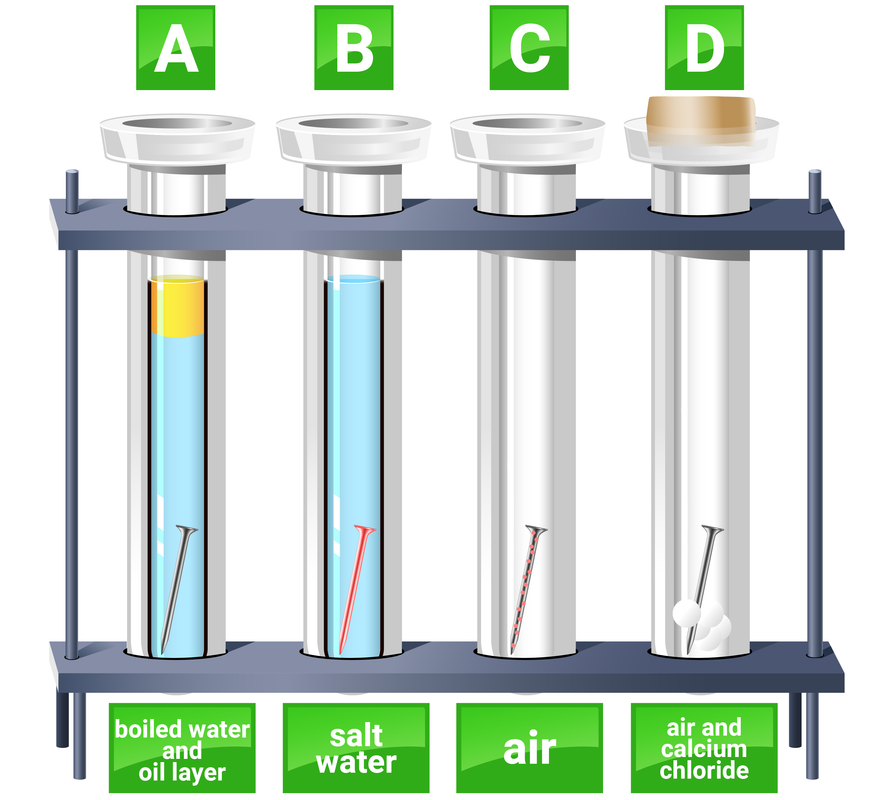 Different conditions demonstrating if an iron nail would corrode.
Different conditions demonstrating if an iron nail would corrode.
Alloys
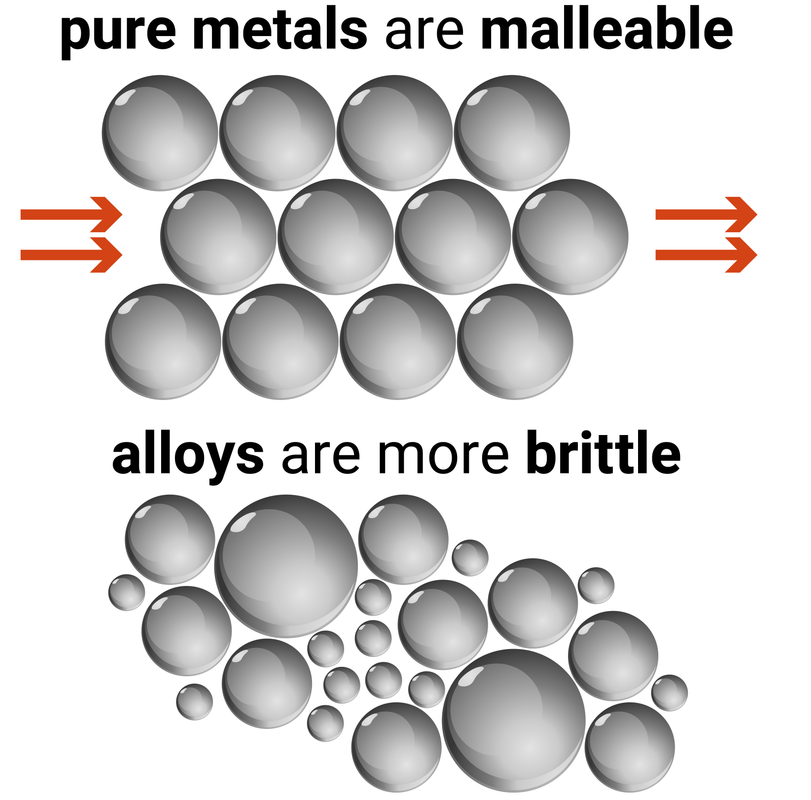
Most metals in everyday use are alloys. An alloy is a mixture of two or more elements, where at least one of the elements is a metal.
Pure metals are generally too soft to use as they have a regular lattice structure. When a force is applied to the metal, the regularly aligned layers are able to easily slide over each other (this makes them malleable).
Alloys are made of different sized atoms, distorting the regular layers so that they are not able to easily slide over each other any more - increasing the strength of the metal (they are less malleable, and more brittle).
Alloy steels
Iron is able to be alloyed with other elements to produce a variety of alloy steels. These alloys have different properties depending on which elements, and how much of each element, is present.
| steel | element | properties | use |
|---|---|---|---|
| low carbon | carbon | softer, more easily shaped | car body parts |
| high carbon | carbon | strong, but brittle | railways, cutting tools |
| stainless | chromium | hard, resistant to rusting | taps, cutlery |
Uses of metals and alloys
Aluminium, gold and copper are useful metals, and the uses of these metals is related to their properties.
Aluminium does not react with water, as its surface is protected by a naturally formed layer of aluinium oxide (allows the metal to resist corrosion). Aluminium foil is used domestically to wrap/store food, because:
- it does not react with the substances in food
- it is malleable and so can easily be folded around the food
- it has a low density so it is very lightweight
Aluminium, gold and copper can also be useful as alloys, some of which are shown below:
| alloy | composition |
|---|---|
| magnalium | aluminium and magnesium |
| brass | copper and zinc |
| jewellery gold | gold and copper |
Magnalium is stronger than pure aluminium, but it has a low density so it used to make aircraft parts.
Ceramics, Polymers and Composites
Different materials have different properties, and the use of the materials is dependant on these properties.
| glass | clay | metal | polymers | |
|---|---|---|---|---|
| appearance | transparent | opaque and dull | opaque and shiny | transparent or opaque |
| melting point | high | high | high | high or low |
| malleable or brittle? | brittle | brittle | malleable | malleable or brittle |
| conductor? | poor | poor | good | poor |
Glass ceramics are made by melting sand with other substances (normally metal oxides), then allowing the liquid to cool and solidify.
Clay ceramics are made by heating clay to high temperatures, causing crystals to form and join. Clay ceramics are often normally then coated with a glaze, which hardens on heating. Types of clay ceramic include brick, china and porcelain.
Polymers can be transparent or opaque and they are often tough and flexible, but some are hard and brittle.
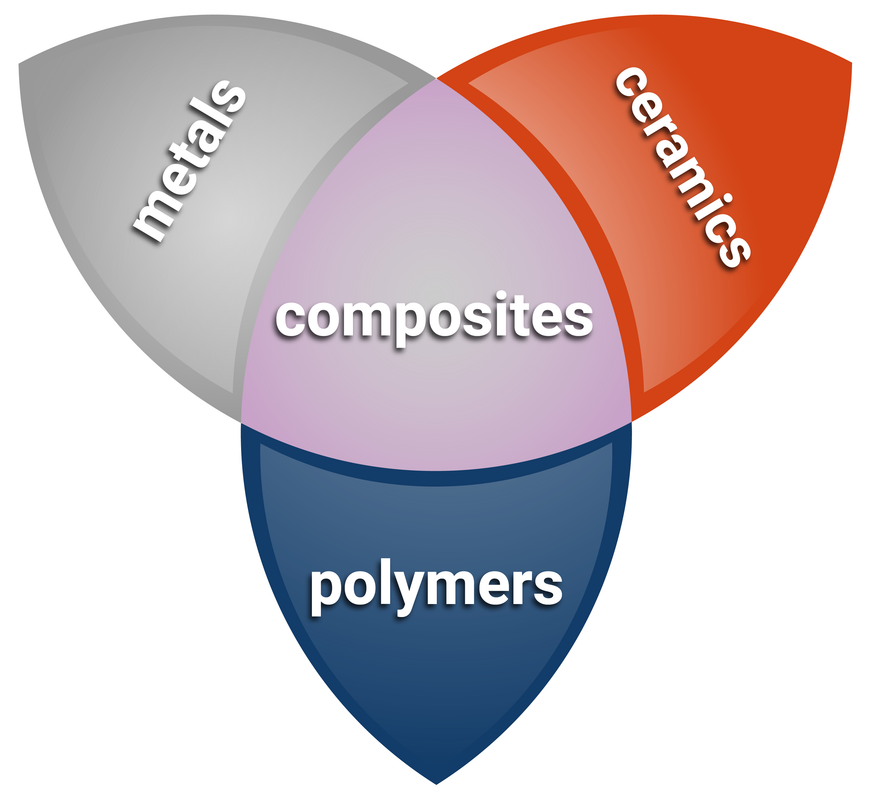
Composites
A composite material consists of two or more materials with different properties to make a new material with more suitable properties. Most composite materials have two components:
- the reinforcement, the substance that makes up the bulk of a composite material
- the matrix, which binds the reinforcement together
Properties of Polymers
The properties of polymers depend on what monomers they are made from and the conditions under which they are made. For example, low density (LD) and high density (HD) poly(ethene) are both produced from ethene.
Low density poly(ethene) has a branched structure meaning that the molecules are arranged randomly.
High density poly(ethene) has less (or no) branching of the polymer chains, so the molecules line up closer together.
Thermosoftening and thermosetting
Thermosoftening plastics melt when heated, meaning that they can be recycled, as this involves melting them before making a new product. This is because thermosoftening plastics do not have branches (made of covalent bonds) between polymer molecules, so the molecules are able to move over each other when heated.
Thermosetting plastics don't melt when heated, and just tend to char/burn instead. However, they are more resistant to higher temperatures than thermosoftening plastics so are used to make electrical plugs.
| polymer | properties | uses |
|---|---|---|
| low density poly(ethene), LDPE | flexible, unreactive, can be made into films | carrier bags, bubble wrap |
| high density poly(ethene), HDPE | strong, flexible, resists shattering, resists chemical attack | plastic bottles, pipes, buckets |
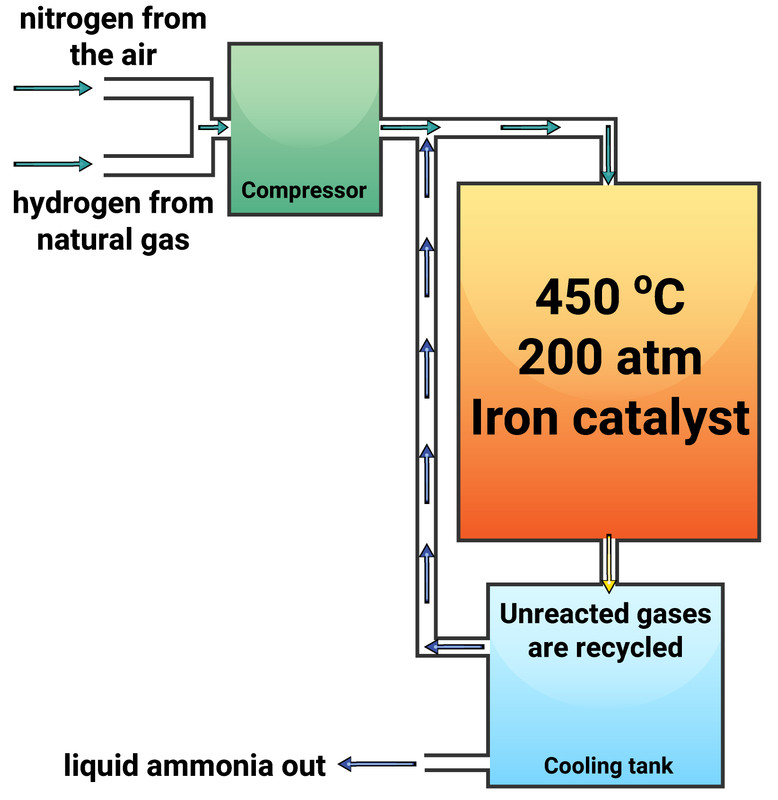
The Haber Process
nitrogen + hydrogen ⇌ ammonia
N2 + 3H2 ⇌ 2NH3
The Haber Process is used to produce ammonia, which can be used to manufacture nitrogen-based fertilisers. This process is a reversible reaction between nitrogen (extracted from the air) and hydrogen (obtained from natural gas). This reaction can reach a dynamic equilibrium.
The conditions for the Haber Process are:
- temperature of 450 °C
- pressure of 200 atmospheres (200 atm)
- iron catalyst (doesn't change the equilibrium, just increases rate of both forward and backwards reactions)
These conditions are a compromise to keep the costs down, but also to be the right conditions to achieve an acceptable yield.
Higher Tier
During industrial reactions, including the Haber process, conditions used are related to:
- the availability and cost of raw materials and energy supplies
- the control of temperature, pressure and catalyst used produce an acceptable yield in an acceptable time
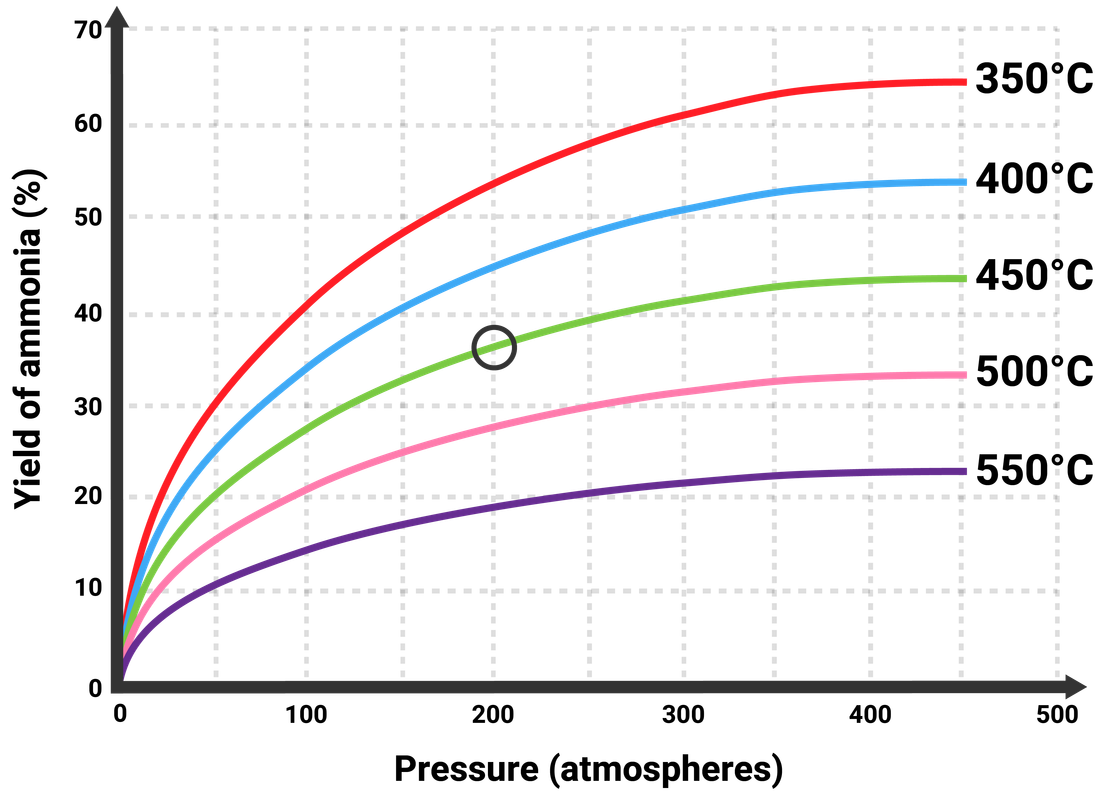
Temperature of 450 °C
- forward reaction is exothermic, so a lower temperature favours the forward reaction to maximise yield
- through heating particles will have more energy, so more frequent, successful collisions between particles (so a faster rate)
- a compromise is made on the temperature
Pressure of 200 atm
- the forward reaction turns 4 moles ('volumes') of gas into 2 moles of gas
- increasing the pressure will favour the forward reaction to maximise the yield (as this forces the particles to take up less volume)
- to maintain this high pressure, lots of energy is needed - so high costs
NPK Fertilisers
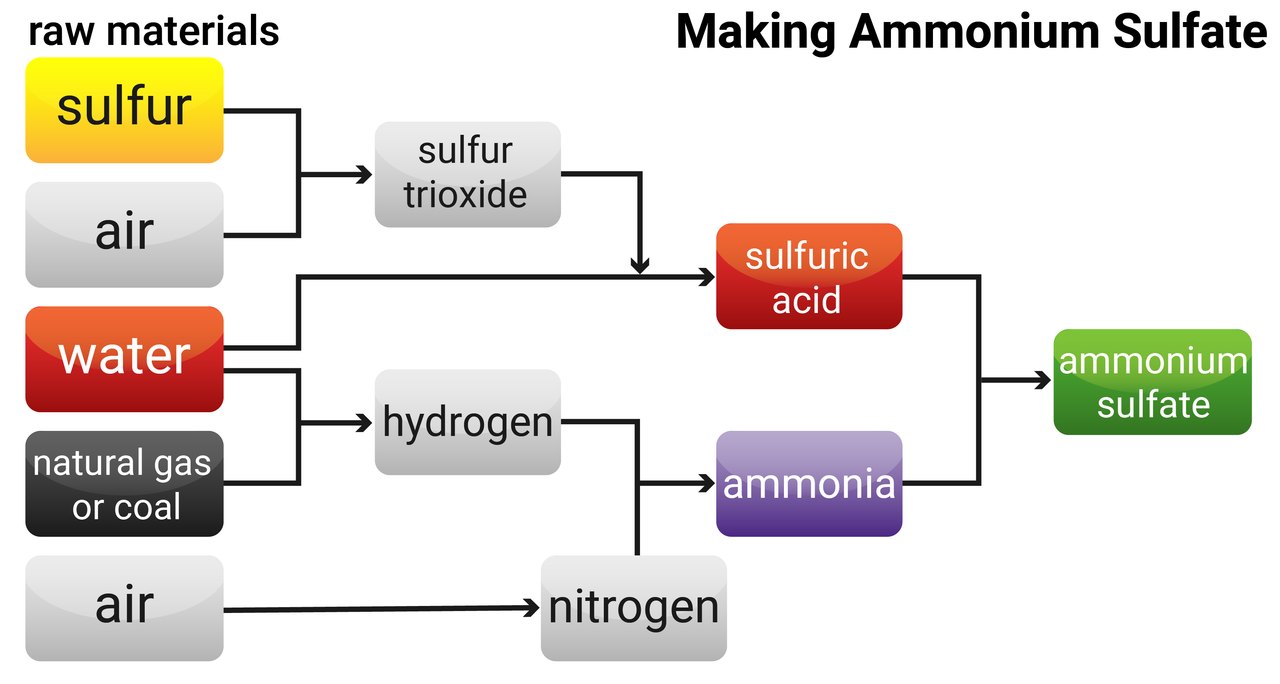
Fertilisers are made from compounds of nitrogen (for leaf development), phosphorus (aids root growth) and potassium (vital for disease resistance and root growth), and are used to improve agricultural productivity. NPK fertilisers contain compounds of all three elements. Industrial production of NPK fertilisers can be achieved using different raw materials in several different steps. NPK fertilisers are formulations of salts containing defined percentages of these elements.
Ammonia is made in the Haber process, and is used to manufacture ammonium salts as well as nitric acid.
Potassium chloride, potassium sulfate and phosphate rock are obtained by mining, but phosphate rock cannot be used directly as a fertiliser.
Phosphate rock is treated with nitric acid or sulfuric acid to produce soluble salts that can be used as fertilisers. Reacting phosphate rock with:
- nitric acid makes phosphoric acid and calcium nitrate
- sulfuric acid makes single superphosphate (this is a mixture of calcium phosphate and calcium sulfate)
- phosphoric acid makes triple superphosphate (calcium phosphate)
Making ammonium sulfate in the lab
Ammonium sulfate can be made in the lab using dilute ammonia solution and dilute
sulfuric acid. Both reactants are soluble, so a titration must be used to carry out this reaction:
ammonia + sulfuric acid → ammonium sulfate
2NH3(aq) + H2SO4(aq) → (NH4)2SO4(aq)
The lab preparation of ammonium sulfate is a batch process. This means that only a small amount of product at any one time, and the apparatus needs to be cleaned before each new batch.
Method
- pour dilute sulfuric acid into a beaker
- add a few drops of methyl orange indicator
- add dilute ammonia solution drop by drop, stirring in between each addition
- continue step 3 until the colour permanently changes from red to orange
- pour the reaction mixture into an evaporating basin, and heat carefully over a boiling water bath
- stop heating before all the water has evaporated. Leave aside for crystals to form
- pour away excess water and leave the crystals to dry in a warm oven (or pat dry with filter paper)
| Laboratory preparation |
Industrial preparation |
|
|---|---|---|
| scale | small | large |
| starting materials |
ammonia solution and dilute sulfuric acid | raw materials for making ammonia and sulfuric acid |
| stages | titration, then crystallisation | many |
| process | batch | continuous |
Industrial production of ammonium sulfate
The industrial production of ammonium sulfate happens on a much larger scale than its production in the lab. A fertiliser factory begins with the raw materials needed to make ammonia and sulfuric acid, rather than buying these two reactants from elsewhere.
The industrial production of ammonium sulfate is a continuous process. The product is made quickly all the time, as long as raw materials are provided.