
Chemical Bonds, Ionic, Covalent and Metallic
Bonding, Structure, and the Properties of Matter
States of Matter
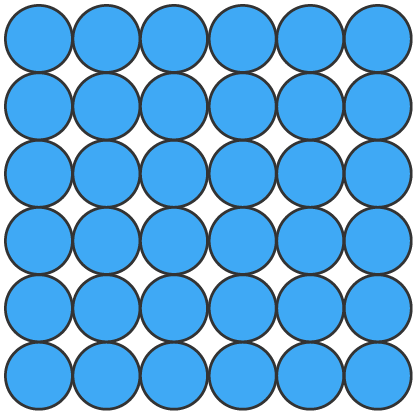
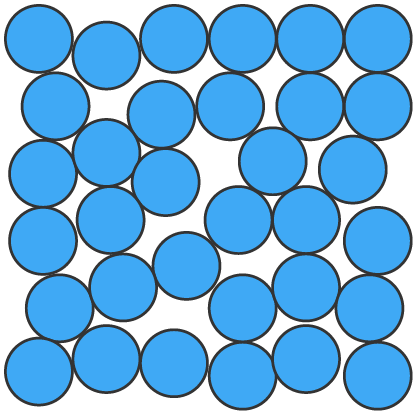
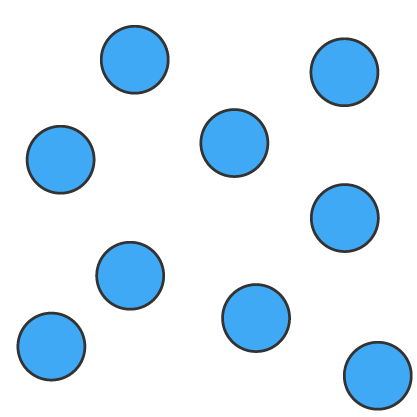
Solids (s), liquids (l) and gases (g) can be represented using particle diagrams. In these diagrams, particles are represented by solid spheres.
Particles in solids have less energy than liquids, which in turn have less energy than gases. To get from a solid to a liquid, or a gas, energy has to be supplied - usually through heating. During these changes the particles gain energy, which is used to break or overcome the intermolecular forces of attraction.
Fluids (liquids and gases) are able to take the shape of their containers, however, only gases can be compressed as their particles are far apart and have space to move into.
The amount of energy required to change state depends on the strength of the intermolecular forces between the particles of each substance. Some of the forces need to be broken during melting, whereas all of the forces must be broken during evaporating/boiling. Changing from one state to another is a physical change as you end up with the same chemical as you began with (whereas a chemical change would lead to different chemicals).
State changes
Solids can turn into liquids through the process of melting. This involves increasing the energy of the particles normally by heating. Liquids can be turned back into solids by freezing, lowering the amount of energy particles have.
Liquids can turn into gases by evaporation or boiling (again by increasing the energy of the particles). Gases can be turned back into liquids by condensation, lowring the amounf of energy particles have.
Some solids can turn straight into a gas, skipping the liquid phase; this is called sublimation.
| Solids | Liquids | Gases |
|---|---|---|
| particles are very close | particles are close | particles are far apart |
| particles are arranged in a regular pattern | particles are randomly arranged | particles are randomly arranged |
| particles vibrate around a fixed position | particles are able to move around each other | particles move quickly in all directions |
| particles have low energy | particles have more energy than solids | particles have more energy than liquids |
 |
 |
 |
Chemical Bonds
There are three types of strong chemical bonds: ionic, covalent and metallic. For ionic bonding the particles are oppositely charged ions. For covalent bonding the particles are atoms which share pairs of electrons. For metallic bonding the particles are atoms which share delocalised electrons.
Ionic bonding occurs in compounds formed from metals combined with non-metals.
Covalent bonding occurs in most non-metallic elements and in compounds of non-metals.
Metallic bonding occurs in metallic elements and alloys.
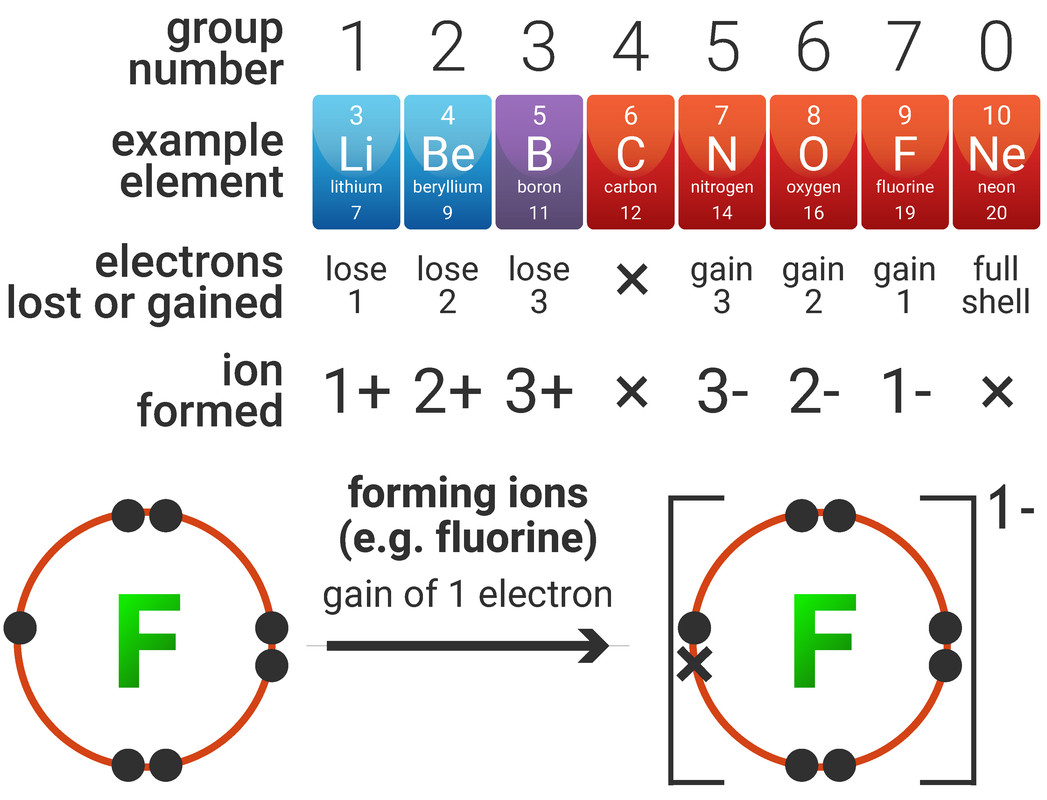
Forming Ions
An ion is an atom (or group of atoms) with a positive or negative charge, formed by either losing or gaining electrons.
Electrons are negatively charged, and protons are positively charged. Atoms are neutral overall, due to the fact they have equal numbers of protons and electrons. By changing the number of electrons (either by an atom losing, or gaining electrons) - the atom forms an ion.
Metals generally form positive ions as it is easier for them to lose electrons to reveal a full shell of electrons, and non-metals generally form negative ions as it is easier for them to gain electrons to make a full shell:
- for example a group 2 metal will form a 2+ ion by losing two negatively charged electrons, forming a positive ion (cation); we say that it has been oxidised
- for example a group 6 non-metal will form a 2- ion by gaining two negatively charged electrons, forming a negative ion (anion); we say that it has been reduced
Common ions and their formulas
| ion | name | example compound |
|---|---|---|
| O2- |
oxide | magnesium oxide (MgO) |
| OH⁻ |
hydroxide | lithium hydroxide (LiOH) |
| F⁻, Cl⁻, Br⁻, I⁻ |
halide | sodium chloride (NaCl) |
| NO3⁻ |
nitrate | potassium nitrate (KNO3) |
| CO32- |
carbonate | calcium carbonate (CaCO3) |
| SO42- |
sulfate | beryllium sulfate (BeSO4) |
Ionic Bonding
Ionic compounds can be described as having a lattice structure that consists of a regular arrangement of oppositely-charged ions held together by strong electrostatic forces. Usually a metal ion and a non-metal ion come together to form an ionic substance.
Ionic substances:
- have high melting points, as large amounts of energy are needed to break the electrostatic forces of attraction
- can conduct electricity when molten or dissolved in water, as the ions are free to move (the charge can flow)
- cannot conduct electricity when solid, as there are no free moving ions
Rules for naming compounds
If the compound has more than one part to its name (e.g. sodium chloride), then the element furthest to the left in the Periodic Table comes first in the name.
| ending | elements involved | example |
|---|---|---|
| -ide | one metal and one non-metal | magnesium sulfide (MgS) |
| -ate | one metal, one non-metal, and oxygen | magnesium sulfate (MgSO₄) |

When drawing a dot-and-cross diagram for ionic bonds you must remember to include the following:
- square brackets to show that it ions are formed
- the charge of each ion to the top right of the square brackets
- only the outershell electrons (also called valence electrons) need to be drawn (unless otherwise stated)
Covalent Bonding
A covalent bond is formed when a pair of electrons are shared between two atoms (usually non-metals). Each atom must share 1 electron each to form one covalent bond - so each covalent bond is made of 2 electrons.
A substance that contains atoms held together by covalent bonds is referred to as a molecule. Simple molecules are typically around 0.1 nanometers in size.
When drawing a dot-and-cross diagram for covalent bonds you must remember:
- only the outershell electrons (also called valence electrons) need to be drawn (unless otherwise stated)
- each covalent bond is a pair of electrons (one electron from each atom in the bond)
- there can be double bonds (4 shared electrons), or even triple bonds (6 shared electrons)
You need to be able to draw dot-and-cross diragems for the following molecules:
- Hydrogen (H₂)
- Water (H₂O)
- Hydrogen chloride (HCl)
- Methane (CH₄)
- Oxygen (O₂)
- Carbon dioxide (CO₂)
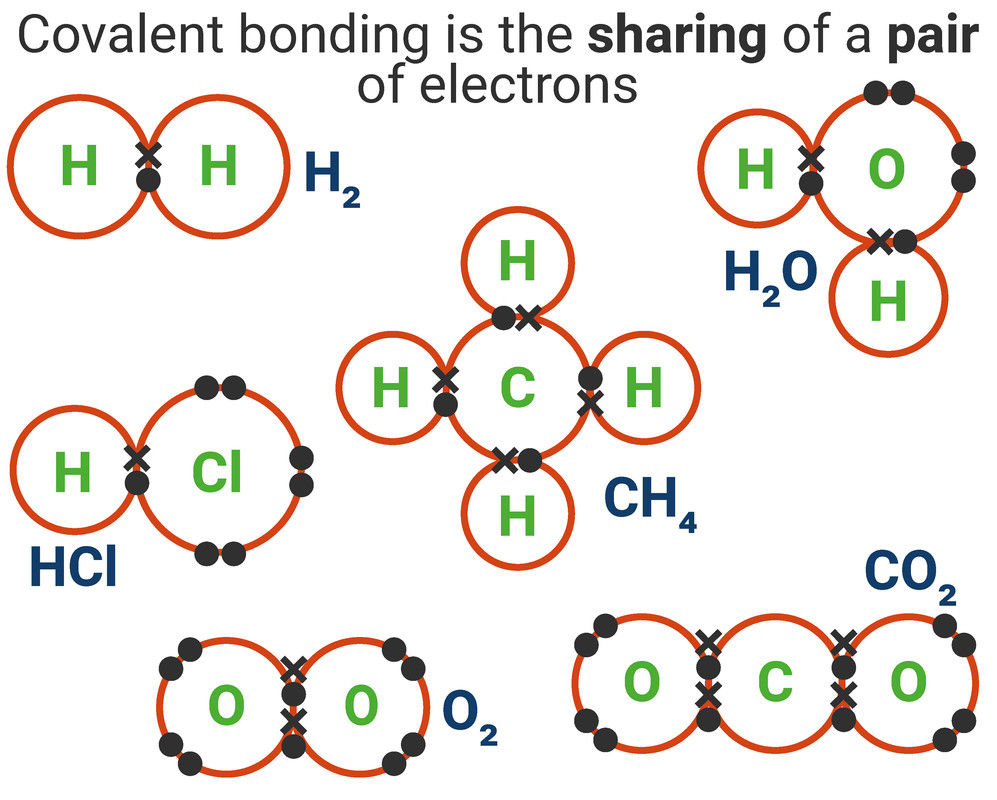
Simple Molecular Compounds
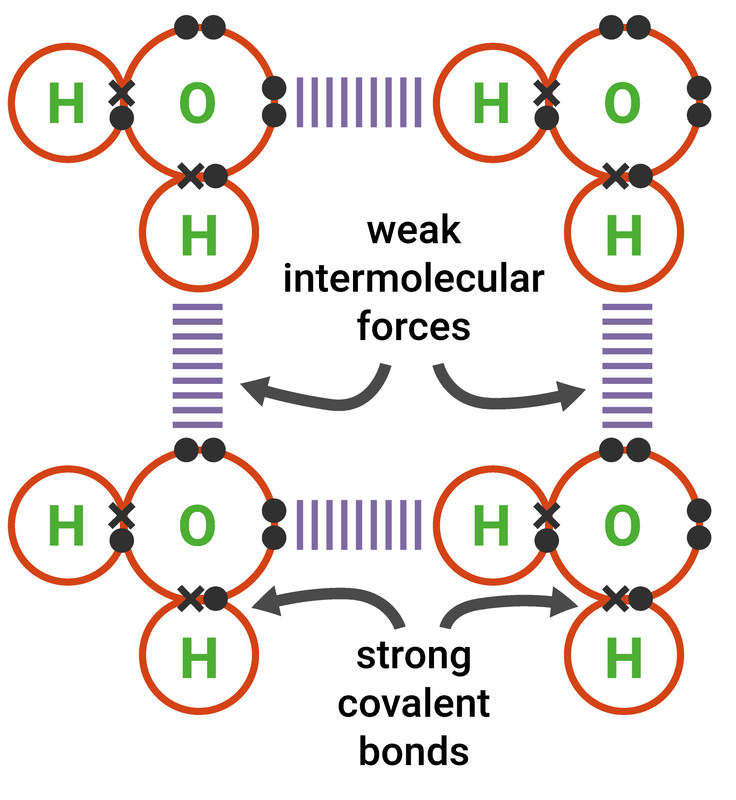
Simple molecular compounds (simple molecules) contain only a few atoms, and we can tell how many of each atom is in the molecule by looking at its formula.
These compounds are usually liquids or gases at room temperature as the molecules are held together by weak intermolecular forces of attraction (but the atoms in the compounds are held together internally by strong covalent bonds), so only a small amount of energy is required to change state. This means simple molecules often have low melting and boiling points.
Most molecular substances are insoluble (or only just soluble) in water. Those which do dissolve often react with the water. Simple molecules do not conduct electricity as there are no free moving electrons or ions.
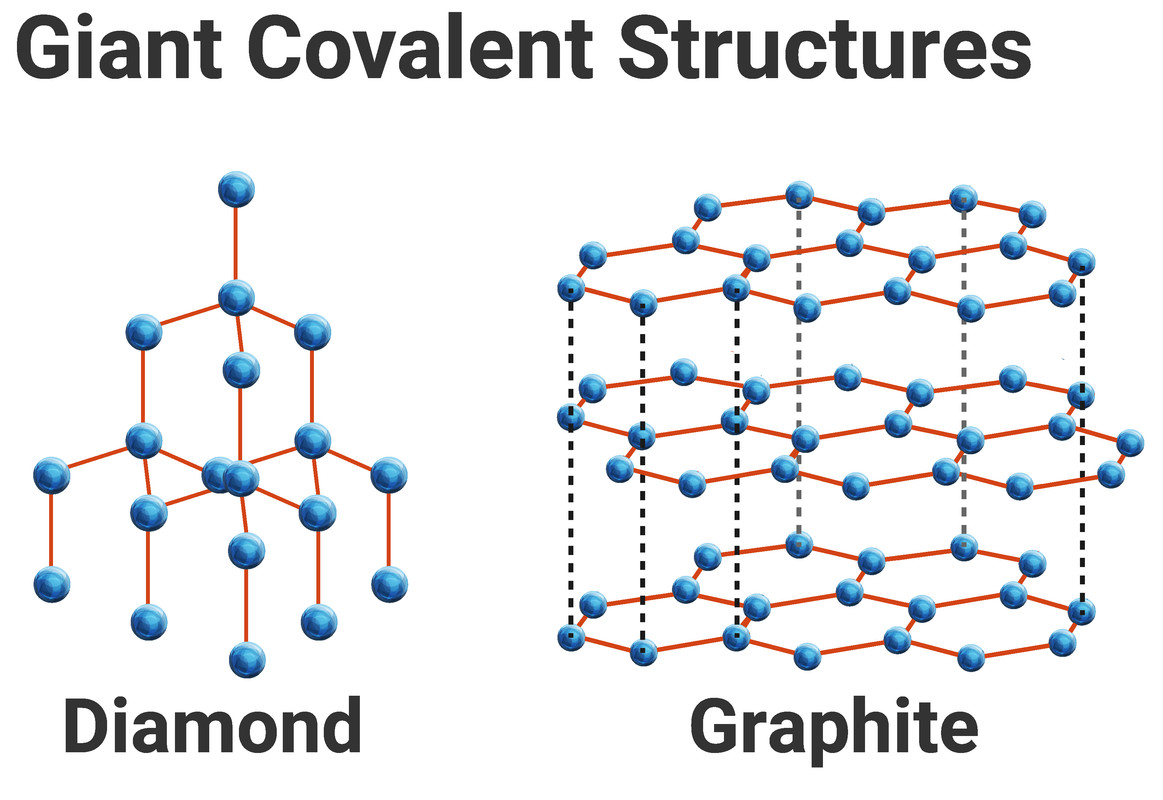
Diamond and Graphite
Graphite and diamond are examples of giant covalent structures.
These compounds are solid at room temperature, because all of the atoms in a giant covalent structure are held together by strong covalent bonds. These bonds have to be broken by large amounts of energy leading to high melting and boiling points. These substances are not soluble in water.
Graphite
Graphite is made from layers of hexagonal rings of carbon, with each atom forming three strong covalent bonds to other carbon atoms. Each atom has a 'spare' electron, not used for bonding, which it contributes to the “sea of delocalised electrons”, thereby being able to conduct heat and electricity well. This is why graphite is often used for electrodes in electrolysis.
Weak forces of attraction hold the layers of graphite together, so they can slide over each other, making graphite a great lubricant.
Diamond
Every carbon atom is strongly covalently bonded to four others in diamond, and because of this it forms a 3D lattice, called a tetrahedron. No free electrons exist in this structure, so it does not conduct electricity.
Diamonds are used as cutting tools as they are the hardest naturally occurring substance due to the arrangement of carbon atoms all bonded by strong covalent bonds.
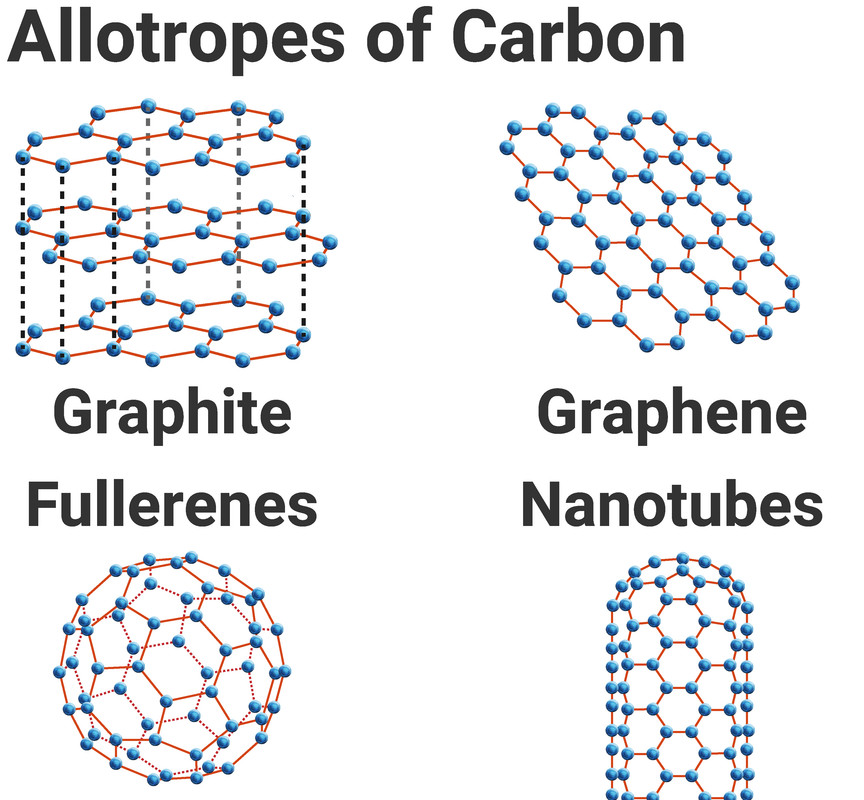
Other Allotropes of Carbon
Carbon can form many different structures with different properties, and when elements can do this - we call them allotropes.
Graphene is just one atom thick (a single layer of graphite). It also contains free moving electrons, and so is very good at conducting electricity.
Fullerenes (such as C₆₀) are spheres or squashed spheres of carbon atoms. They are made up of large molecules, but do not have a giant covalent structure. Weak intermolecular forces exist between individual fullerenes. Little energy is needed to overcome these forces, so substances consisting of buckyballs are slippery and have lower melting points than graphite or diamond.
A nanotube resembles a layer of graphene, rolled into a tube shape. Nanotubes have high tensile strength, so they are strong in tension and resist being stretched. Like graphene, nanotubes are strong, and they conduct electricity because they have delocalised electrons.
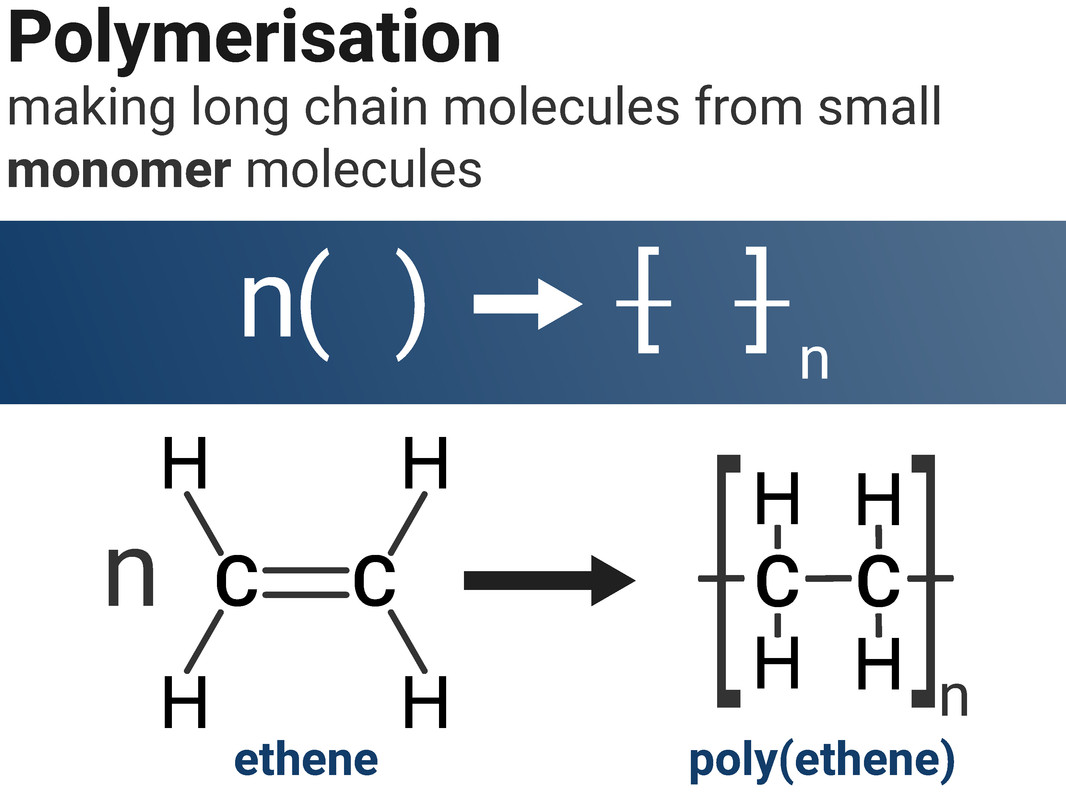
Polymers
Polymers are large molecules, made of ‘repeating units’ called monomers.
All the atoms in a polymer are bonded to other atoms to make a long chain of strong covalent bonds, usually with a carbon backbone.
Because polymers can be very long (thousands or millions of atoms!) we don't write out their full structure - and instead we can show their repeating units in a diagram similar to the one shown. This shows how we can turn ethene into poly(ethene).
The "n" shows that there are any number of ethene molecules on the left, and that the same number of atoms exist on the left-hand side of the reaction as do in the product on the right.
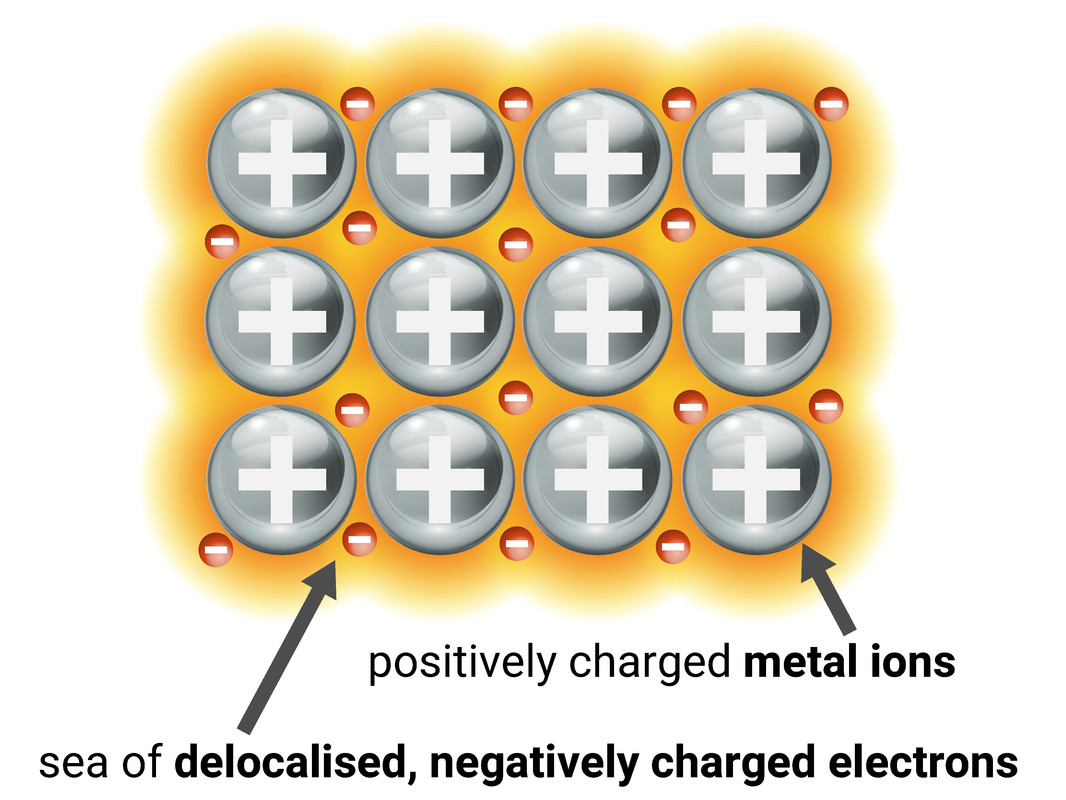
Metallic Bonding
Metals have some unique properties - and it's all to do with how they bond! Their structure is formed from positive metal ions held together by a “sea of delocalised electrons” from the outer shells of the metal atoms. The electrons are free to move around from each atom to atom as they please.
The strong electrostatic forces between the ions and electrons mean metals have very high melting points (large amounts of energy are needed to break these forces).
Because the electrons are able to move freely, it means metals are good conductors of electricity and heat.
Metals are also shiny, as well as malleable (bendable) and ductile (can be drawn into wires) as they have regular layers of atoms. These layers can slide over each other if they are hammered.
In pure metals, atoms are arranged in layers, which allows metals to be bent and shaped. Pure metals are too soft for many uses and so are mixed with other metals to make alloys which are harder.
Non metals:
- are dull (not shiny)
- have low melting and boiling points
- are poor conductors (good insulators)
Alloys (mixtures of metal atoms and other elements) are less malleable than pure metals as they have irregular layers (made up of different sized atoms), and so they cannot slide over each other as easily. The atoms are still held together by metallic bonding.
Bonding Models
We use models to help us represent what molecules look like.
Dot and cross diagrams don't show:
- Ionic Bonding
- the lattice structure
- ionic bonds
- Covalent Bonding
- the relative sizes of the atoms
- the intermolecular forces
Chemical formulas don't show:
- Ionic Bonding
- the lattice structure
- the charges on the ions
- Covalent Bonding
- bonding in the molecule
- the shape of the molecule
Examples include:
- hydrogen gas (H2)
- carbon dioxide (CO2)
- water (H2O)
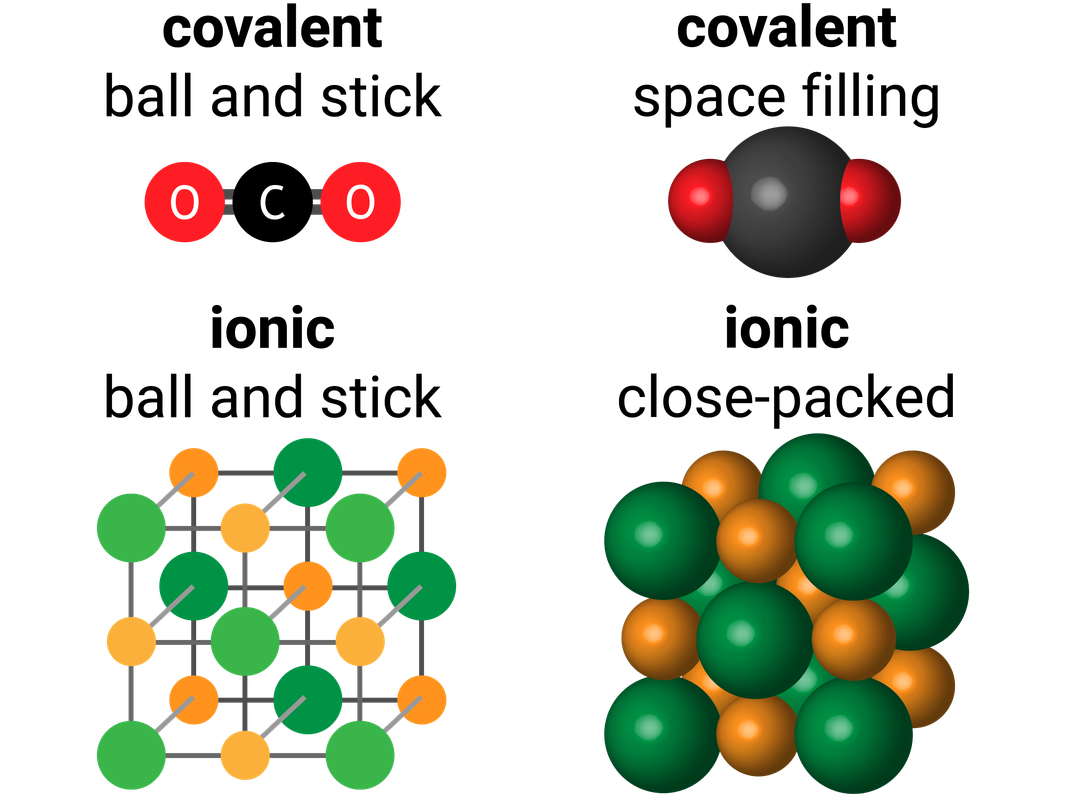
Space Filling (covalent) or Close Packed (ionic) don't show:
- Ionic Bonding
- how the ions were formed
- more than 1 or 2 layers
- Covalent Bonding
- how covalent bonds are formed
- which elements are present, unless a colour key is given
Ball and Stick diagrams don't show:
- Ionic Bonding
- the charges of the ions
- that there aren't actually spaces between ions
- Covalent Bonding
- how covalent bonds are formed
- the bonds are forces, not sticks
Straight Lines (covalent) don't show:
- the size of the atoms
- electron densities
Examples include:
- hydrogen gas H-H
- carbon dioxide O=C=O
- water H-O-H