Reactivity of Metals
Chemical Changes
Reactivity Series
When metals react with other substances the metal atoms form positive ions. The reactivity of a metal is related to its tendency (how likely it is) to form positive ions.
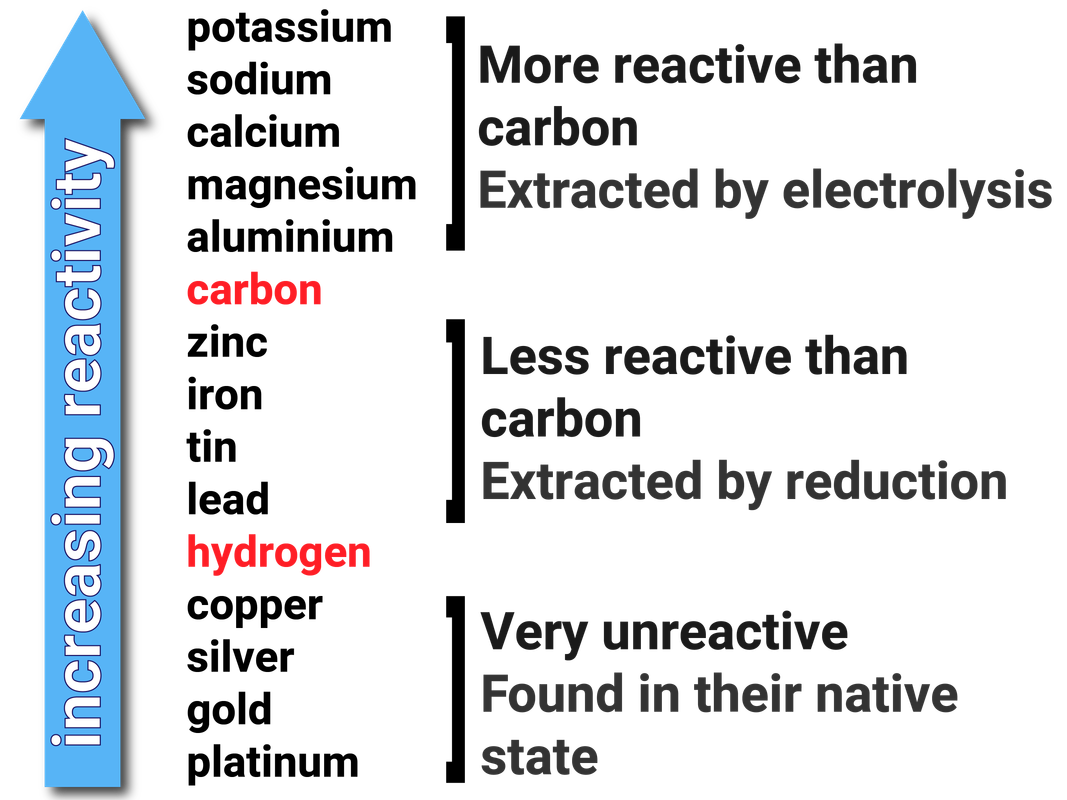
Metals can be arranged in order of their reactivity in a reactivity series. The metals potassium, sodium, lithium, calcium, magnesium, zinc, iron and copper can be put in order of their reactivity from their reactions with water and dilute acids.
The non-metals hydrogen and carbon are often included in the reactivity series to give an indication about how the metals can be extracted.
Metals less reactive than hydrogen are generally found in their elemental form in the Earth's crust (native state). They do not need to be extracted.
Metals less reactive than carbon can be extracted by heating the metal with carbon. As the carbon is more reactive, it will displace the metal in its ore - reducing the metal.
Metals more reactive than carbon have to be extracted using electrolysis. This is very costly, in terms of both money and energy.
Reactions of Metals
All metals will react with substances differently, and even the same metal can be more or less reactive depending on what it is being reacted with.
Rather than having multiple tables for each specific reaction, a diagram was created to show the average reactivity of metals with water and dilute acids.
The reactivity series can also be used to see the relative tendency for metal atoms to form cations - the higher up the series, the more likely it will form a positive ion.
Something similar can be said for a metal's resistance to oxidation - the higher up the series, the more easier it is to oxidise.
| metal | reaction with water | reaction with dilute acid |
|---|---|---|
| potassium | react with cold water to form hydrogen and a metal hydroxide | react violently |
| sodium | ||
| calcium | react to form hydrogen and a salt solution | |
| magnesium | react very slowly, if at all, with cold water - but will react with steam to form hydrogen and a metal oxide | |
| aluminium | ||
| zinc | ||
| iron | ||
| copper | react with cold water to form hydrogen and a metal hydroxide | do not react |
| silver | ||
| gold |
REDOX (Reduction and Oxidation)
Most metals do not exist naturally on their own, but combined with other elements, and compounds, as ores in the Earth's crust. Most ores contain oxygen, and so when a metal reacts with oxygen a metal oxide is formed. This is called oxidation.
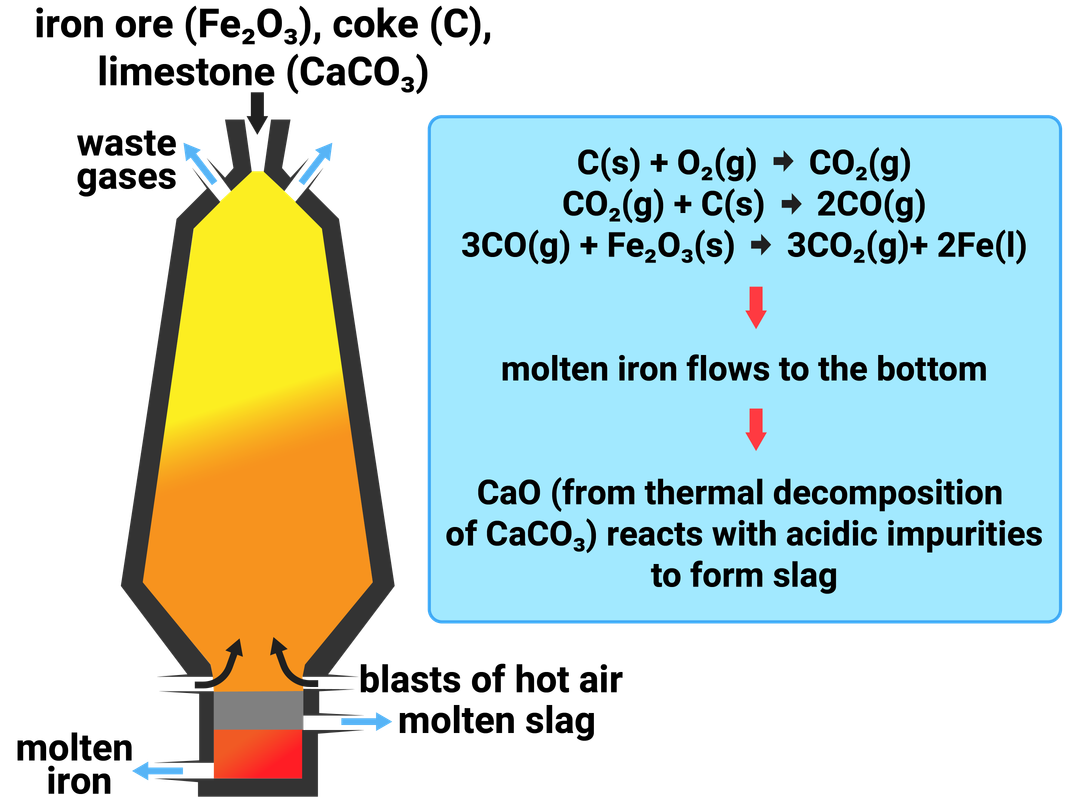
Removing the oxygen from the compound to extract the metal is called reduction. To extract iron we need to reduce the ore. This means we take away the oxygen.
Iron is naturally found as iron oxide, so we can reduce the ore by heating it with a reducing agent. By adding carbon monoxide, we can remove the oxygen from the ore to make iron and carbon dioxide:
iron oxide + carbon monoxide → iron + carbon dioxide
Fe2O3 + CO → Fe + CO2
The metals right at the bottom of the reactivity series are unreactive, and are found in their native state as uncombined elements in the Earth's crust.
Knowledge of the blast furnace (shown here) is not needed, and is only shown to illustrate how carbon is used to extract iron.
Extracting Metals
The least reactive metals are found in their native state in the Earth's crust. This means they are found as uncombined elements.
Any metal more reactive than hydrogen, but less reactive than carbon, can be extracted by adding carbon to displace the metal from its oxide (or other compounds). This is known as reduction.
The most reactive metals have to be extracted using electrolysis, as carbon is not reactive enough to displace the metals. This is rather costly, and uses a lot of electricity.
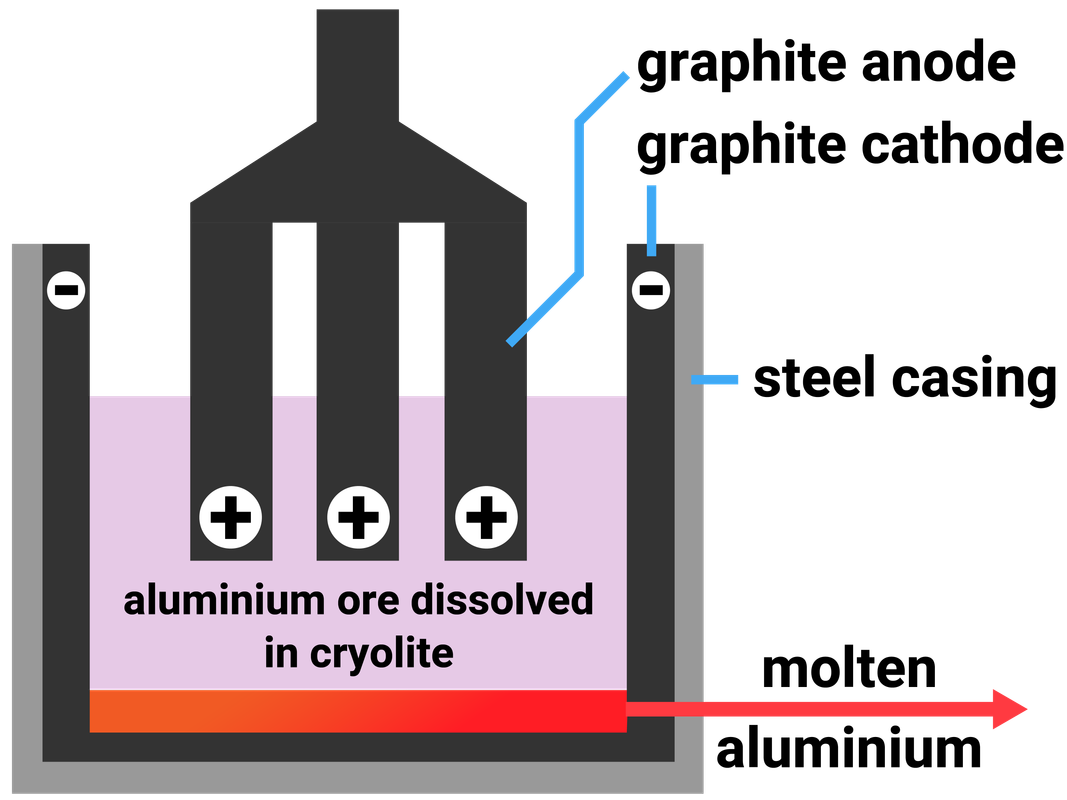
Extracting Aluminium
Aluminium oxide is insoluble in water, so it must be molten to act as an electrolyte. A lot of energy must be transferred to break aluminium's ionic bonds, which is expensive - so to reduce costs, powdered aluminium oxide is dissolved in molten cryolite. This melts at a lower temperature than aluminium oxide and helps reduce costs.
Higher Tier
Half equation at the cathode:
Al3+ + 3e- → Al
Half equation at the anode:
2O2- → O2 + 4e-
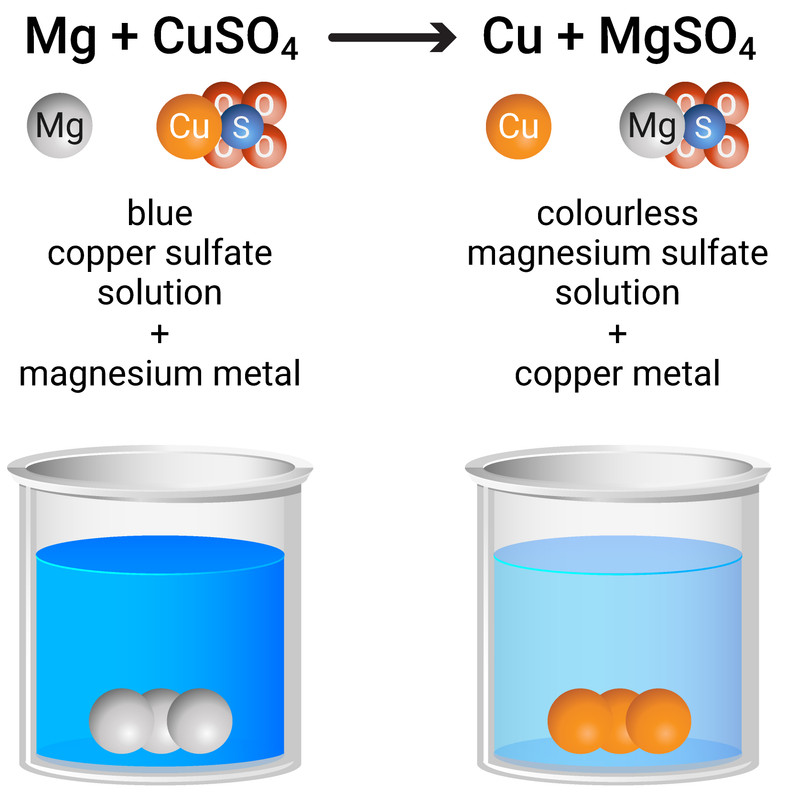
Displacement Reactions
A more reactive metal can displace a less reactive metal from a compound in solution. This is one way to extract metals. For example:
magnesium + copper sulfate → copper + magnesium sulfate
Mg + CuSO4 → Cu + MgSO4
Higher Tier
Ionic equations only show us the ions that change in a chemical reaction. This is useful as it shows what is oxidised and reduced. We can start by writing a balanced symbol equation for a reaction:
Mg(s) + CuSO4(aq) → Cu(s) + MgSO4(aq)
Then we can write out all the ions involved:
Cu²⁺(aq) + SO4²⁻(aq) + Mg(s) → Cu(s) + Mg²⁺(aq) + SO4²⁻(aq)
We can identify the ions that don't change, these are called the spectator ions. These are of no interest in the reaction, so we can ignore them and write out our ionic equation:
Mg(s) + Cu²⁺(aq) → Cu(s) + Mg²⁺(aq)
Once we have an ionic equation, we can take it one step further and look at half equations. These look at how the electrons behave. We can see there are only two different chemicals involved, and we can split our equation in half... to make half equations:
Cu²⁺ + 2e⁻ → Cu
Mg - 2e⁻ → Mg²⁺
By writing our equation in this way, we can immediately see that copper had to gain two electrons (reduced) in this reaction, and those electrons came from magnesium (oxidised).