
Chemical Cells and Fuel Cells (chemistry only)
Energy Changes
Cells and Batteries
Cells contain chemicals which react to produce electricity. The voltage produced by a cell is dependent upon a number of factors including the type of electrode and electrolyte.
A simple cell can be made by connecting two different metals in contact with an electrolyte. Batteries, like the ones in torches and mobile phones, are made up of chemical cells. There are many types of chemical cell, each with different chemical reactions.
If we connect different combinations of metals to make a cell, we find that the voltage changes. In the table we can see the voltage produced when using magnesium as the negative electrode, and zinc and copper as the positive electrodes.
A simple chemical cell is made up of two parts:
- two different metals, each dipped into a solution of one of their salts
- a 'salt bridge' to allow dissolved ions to pass from one solution to the other
There are many types of chemical cell, each with different chemical reactions.
| metal at the positive electrode | voltage produced with magnesium as negative electrode (V) |
|---|---|
| magnesium | 0.00 |
| zinc | + 1.61 |
| copper | + 2.71 |
Batteries
In non-rechargeable cells and batteries the chemical reactions stop when one of the reactants has been used up. Alkaline batteries are non-rechargeable.
Rechargeable cells and batteries can be recharged because the chemical reactions are reversed when an external electrical current is supplied.
Batteries, like the ones in torches and mobile phones, are made up of chemical cells.
Chemical Cells and Fuel Cells
Chemical cells use chemical reactions to convert and transfer energy to electrical energy. They will produce a voltage only up until one of the reactants has been used up (we say the battery has "gone flat").
Fuel cells will produce a voltage continuously, provided they have a constant supply of fuel and oxygen (from the air).
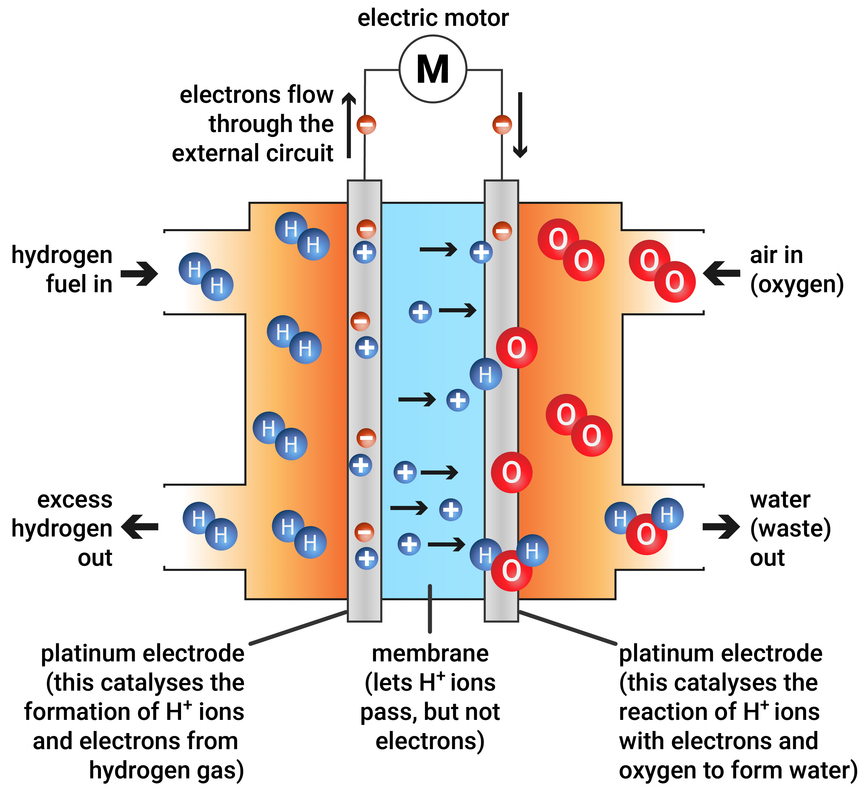
Hydrogen-oxygen fuel cells
In this type of fuel cell, hydrogen and oxygen are used to produce a voltage. The only product from this reaction is water.
A hydrogen-oxygen fuel cell and electric motor are much quieter, and need less maintenance, than a petrol or diesel engine, but the hydrogen still needs to be stored in a container - like a tank.
hydrogen + oxygen → water
2H2 + O2 → 2H2O
Higher Tier
At the cathode: 2H2(g) → 4H+(aq) + 4e-
At the anode: O2(g) + 4H+(aq) + 4e- → 2H2O(l)
Uses of Fuel Cells
Hydrogen, diesel and petrol are all highly flammable fuels. Fuel cells have their advantages and disadvantages depending on the use.
Fuel cells in spacecraft
| strengths | weakness |
|---|---|
| no moving parts to maintain | have to be continuously supplied with oxygen and hydrogen although this could be rectified by using solar cells to electrolyse the water produced back into oxygen and hydrogen |
| small in size for the amount of electricity produced | |
| water they produce can be used for drinking water |
Fuel cells in vehicles
| strengths | weaknesses | |
|---|---|---|
| fuel cells |
|
|
| petrol/diesel |
|
|