Particles and atomic structure
C1: Particles
States of Matter
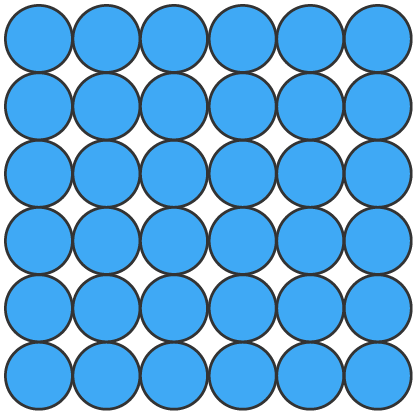
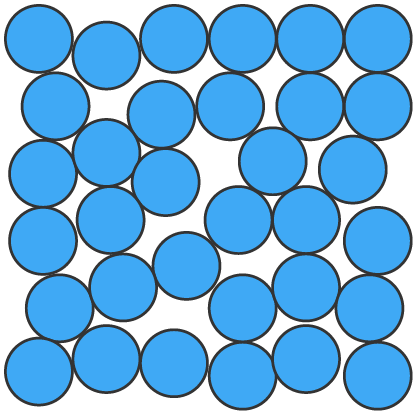
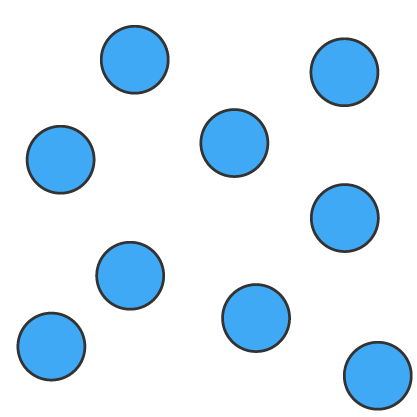
Solids (s), liquids (l) and gases (g) can be represented using particle diagrams. In these diagrams, particles are represented by solid spheres.
Particles in solids have less energy than liquids, which in turn have less energy than gases. To get from a solid to a liquid, or a gas, energy has to be supplied - usually through heating. During these changes the particles gain energy, which is used to break or overcome the intermolecular forces of attraction.
Fluids (liquids and gases) are able to take the shape of their containers, however, only gases can be compressed as their particles are far apart and have space to move into.
The amount of energy required to change state depends on the strength of the intermolecular forces between the particles of each substance. Some of the forces need to be broken during melting, whereas all of the forces must be broken during evaporating/boiling. Changing from one state to another is a physical change as you end up with the same chemical as you began with and the change can be easily reversed, (whereas a chemical change would lead to different chemicals being formed, and are often not easily reversed).
Bulk properties
Individual atoms do not have the physical properties of the substances that contain them. For example, a copper atom cannot conduct electricity, even though a piece of copper can do this. Bulk properties are properties due to many atoms, ions or molecules acting together.
State changes
Solids can turn into liquids through the process of melting. This involves increasing the energy of the particles normally by heating. Liquids can be turned back into solids by freezing, lowering the amount of energy particles have.
Liquids can turn into gases by evaporation or boiling (again by increasing the energy of the particles). Gases can be turned back into liquids by condensation, lowring the amounf of energy particles have.
Some solids can turn straight into a gas, skipping the liquid phase; this is called sublimation.
| Solids | Liquids | Gases |
|---|---|---|
| particles are very close | particles are close | particles are far apart |
| particles are arranged in a regular pattern | particles are randomly arranged | particles are randomly arranged |
| particles vibrate around a fixed position | particles are able to move around each other | particles move quickly in all directions |
| particles have low energy | particles have more energy than solids | particles have more energy than liquids |
 |
 |
 |
Higher Tier
Limitations of the simple model above include that in the model:
- all particles are represented as spheres
- the spheres are inelastic solids
This model does not take into account the forces of attraction between particles, the size of particles and the space between them.
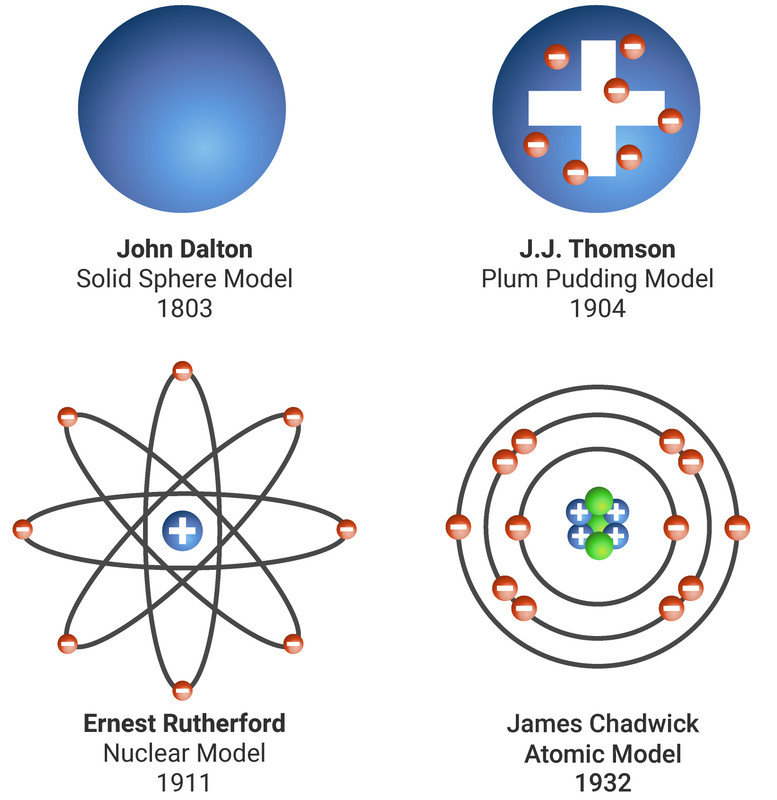
Atomic History
New experimental evidence may lead to a scientific model being changed or replaced.
John Dalton thought that all matter was made of tiny particles called atoms, which he imagined as tiny spheres that could not be divided.
J.J. Thomson discovered the electron. He suggested a plum pudding model of the atom. In this model the atom is a ball of positive charge with negative electrons randomly distributed within it.
Hans Geiger and Ernest Marsden tested the plum pudding model. In the experiment they fired positively charged alpha particles at thin gold foil. Most alpha particles went straight through the foil, but a few were scattered in different directions or were reflected back. Ernest Rutherford explained these results as:
- the mass of an atom is concentrated at its centre, the nucleus (which is very small)
- the nucleus is positively charged
Niels Bohr adapted Ernest Rutherford's model. Bohr did calculations that led him to suggest that electrons orbit the nucleus in fixed energy levels - called shells. The shells are at certain distances from the nucleus.
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles.
James Chadwick found evidence for the existence of particles in the nucleus with mass but no charge. These particles are called neutrons.
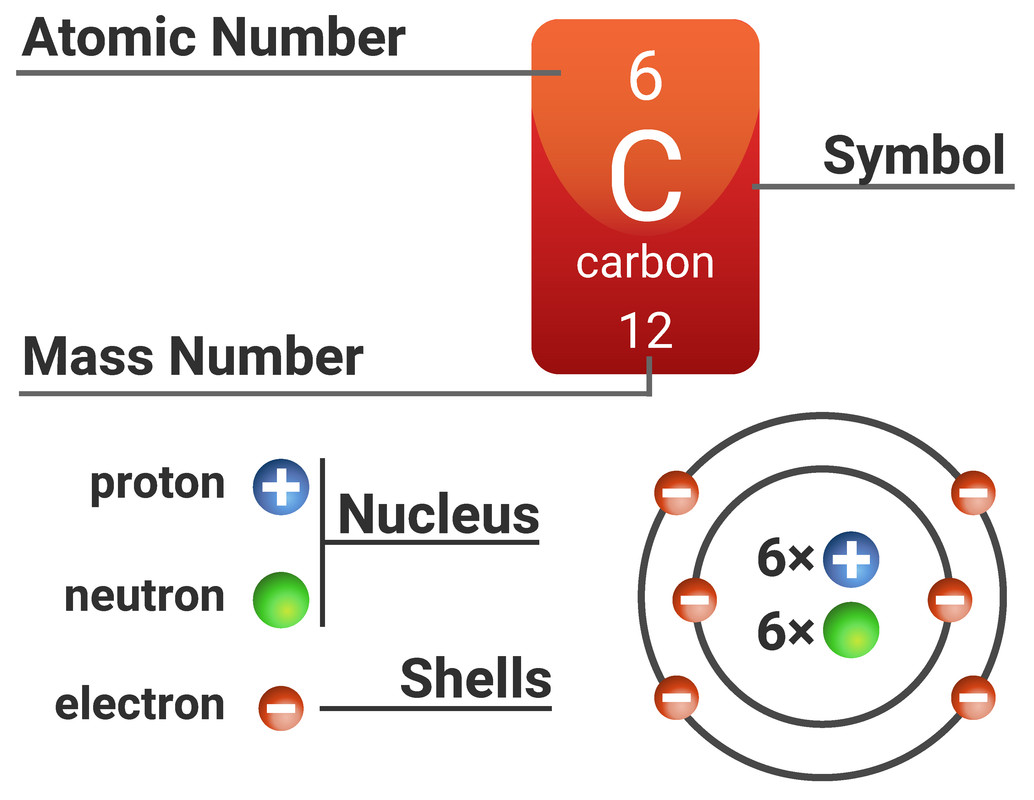
Atomic Structure
Atoms are made from three types of subatomic particles: protons, neutrons and electrons.
On the Periodic Table you will find every element that we know about, which in turn is a list of every atom that we have discovered.
Next to each element's symbol you will find several numbers:
- The atomic number - this is the number of protons
- The mass number - this is the total number of protons and neutrons
| name of particle |
mass (relative) |
charge (relative) |
location in atom |
|---|---|---|---|
| proton | 1 | +1 | nucleus |
| neutron | 1 | 0 | nucleus |
| electron | 0.0005 | -1 | shells |
Every atom of the same element has the same number of protons as electrons. This means that every atom is neutral as it will have the same number of positive charges as negatives.
An atom has a diameter of about 0.0000001 mm (0.1 nm), with about 99% of an atom being empty space! The radius of a nucleus is less than 1/10000 (that's really small!) of that of the atom.
The proton number of an element defines the element (it is unique to that element - e.g. only carbon atoms have 6 protons). Every atom of one element must have the same number of protons, if it has more or less protons it is now an atom of a different element.
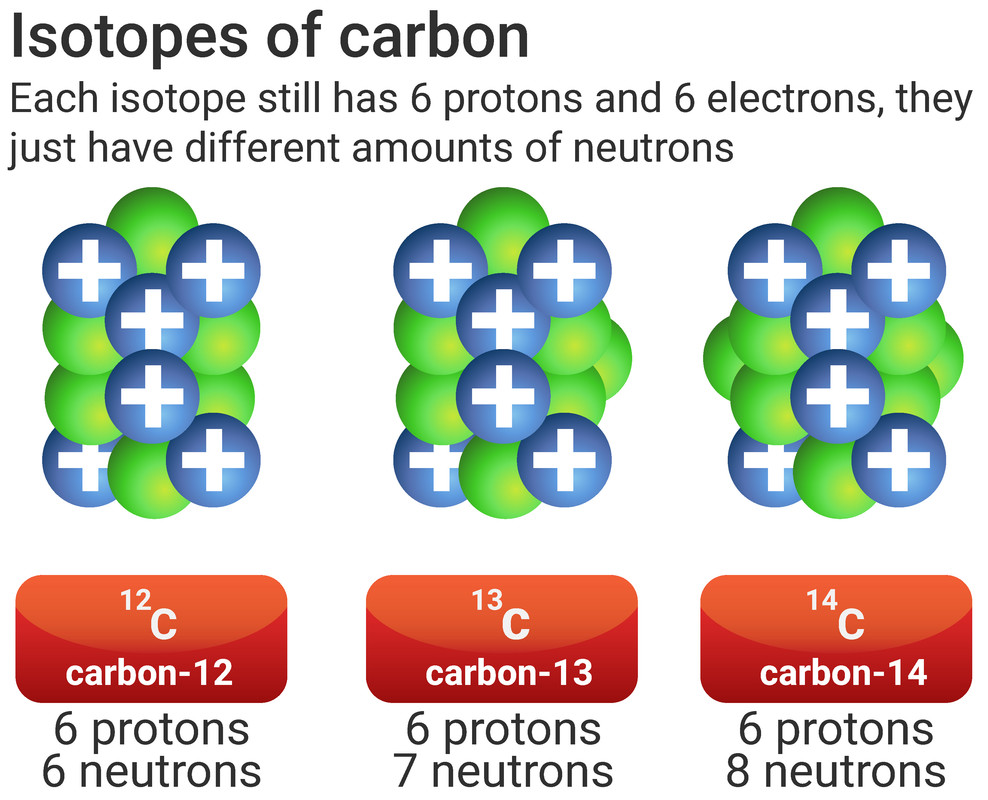
Isotopes
All atoms will always have the same number of protons as they do electrons. This is because atoms are neutral, and this is achieved by having the same number of positive charges (protons) and negative charges (electrons).
Not all atoms will have the same number of neutrons. All atoms of carbon have 6 protons, making its atomic number 6. Most of those carbon atoms will also have 6 neutrons, making the mass number 12 (6+6). However, some of those atoms of carbon will have a different number of neutrons, making the mass number different too.
Isotopes are different forms of the same element, they have:
- the same atomic number (same number of protons and electrons)
- a different mass number (different number of neutrons)
Electron Configurations
Electrons orbit around an atom in a certain pattern. Around the nucleus are different energy levels, often called shells. Each shell can be filled to a specific number of electrons, and these limits are the same for every element. Each shell must be filled before another shell can take electrons.
Atoms will only fill to a maximum of:
- 2 electrons in the first shell,
- 8 in the second shell,
- 8 in the third shell
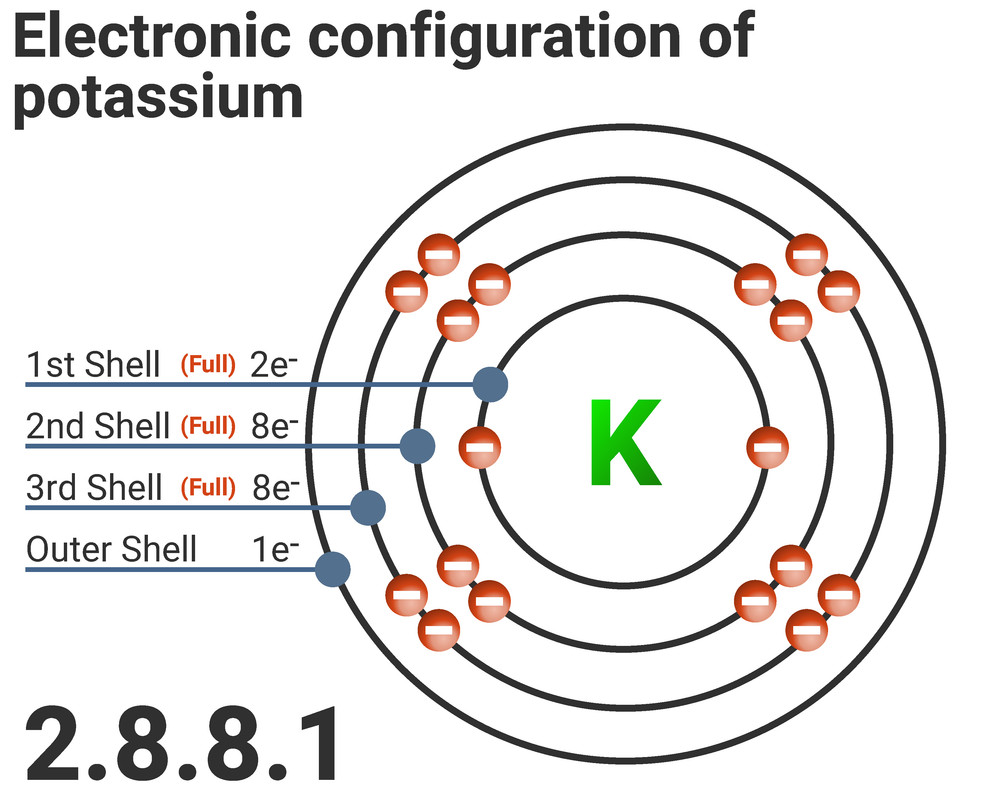
We can either write, or draw, the electron configuration of an atom. Here you can see the electron configuration of potassium.
Potassium:
- is in period 3 - so has 3 shells of electrons
- is in group 1 - so has 1 electron on its outermost shell
- has a configuration of 2.8.8.1 because all shells must be filled before a new shell can contain electrons