States of Matter and Mixtures
States of Matter
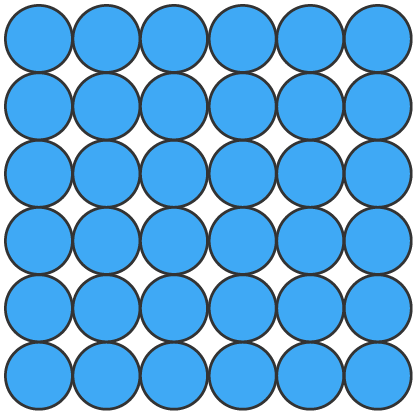
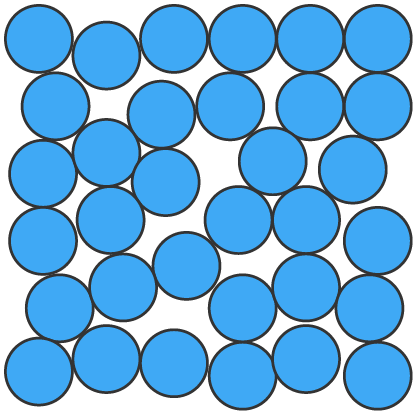
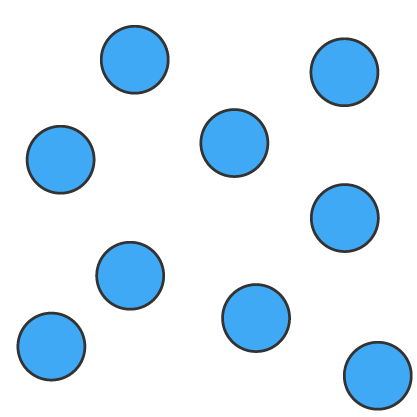
Solids (s), liquids (l) and gases (g) can be represented using particle diagrams. In these diagrams, particles are represented by solid spheres.
Particles in solids have less energy than liquids, which in turn have less energy than gases. To get from a solid to a liquid, or a gas, energy has to be supplied - usually through heating. During these changes the particles gain energy, which is used to break or overcome the intermolecular forces of attraction.
Fluids (liquids and gases) are able to take the shape of their containers, however, only gases can be compressed as their particles are far apart and have space to move into.
The amount of energy required to change state depends on the strength of the intermolecular forces between the particles of each substance. Some of the forces need to be broken during melting, whereas all of the forces must be broken during evaporating/boiling. Changing from one state to another is a physical change as you end up with the same chemical as you began with (whereas a chemical change would lead to different chemicals).
State changes
Solids can turn into liquids through the process of melting. This involves increasing the energy of the particles normally by heating. Liquids can be turned back into solids by freezing, lowering the amount of energy particles have.
Liquids can turn into gases by evaporation or boiling (again by increasing the energy of the particles). Gases can be turned back into liquids by condensation, lowring the amounf of energy particles have.
Some solids can turn straight into a gas, skipping the liquid phase; this is called sublimation.
| Solids | Liquids | Gases |
|---|---|---|
| particles are very close | particles are close | particles are far apart |
| particles are arranged in a regular pattern | particles are randomly arranged | particles are randomly arranged |
| particles vibrate around a fixed position | particles are able to move around each other | particles move quickly in all directions |
| particles have low energy | particles have more energy than solids | particles have more energy than liquids |
 |
 |
 |
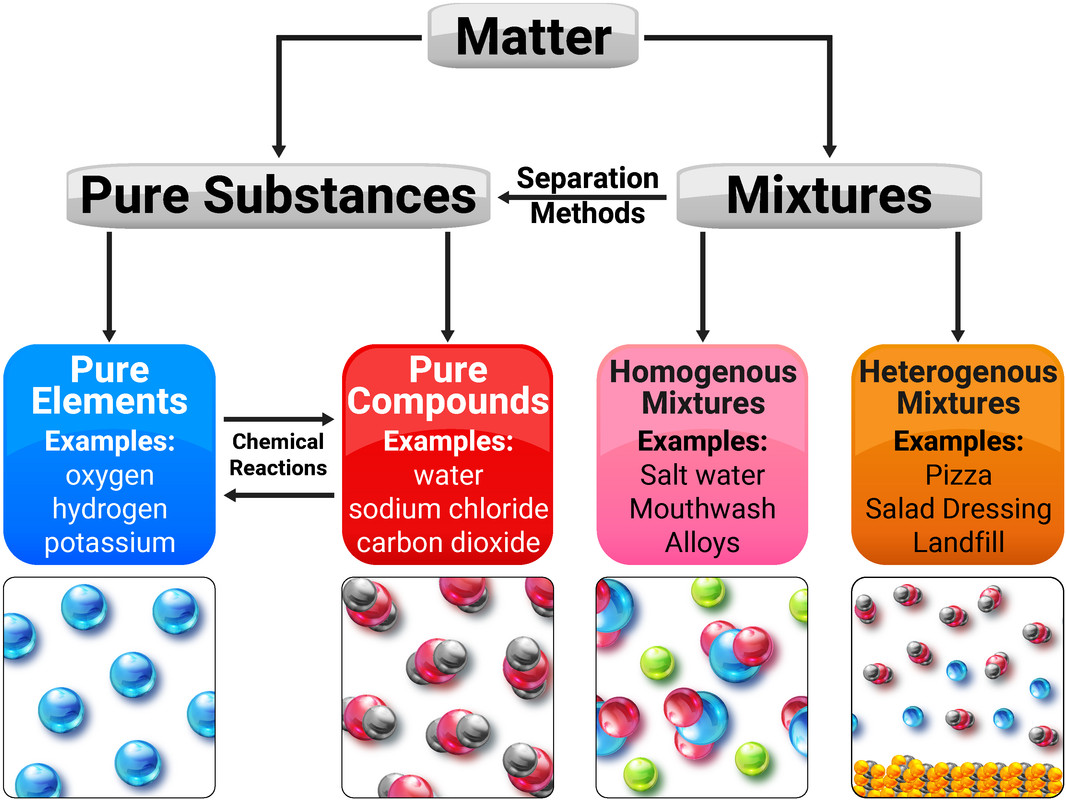
Pure Substances
A pure substance is either a single element, or compound, that is not mixed with any other substance.
A mixture consists of two or more elements/compounds, that are not chemically combined together and can be separated by physical processes.
Pure substances will melt and boil at specific temperatures, so we can use the temperature that a substance melts/boils at to tell how pure it is. Impure substances will melt/freeze or boil/condense over a range of temperatures.
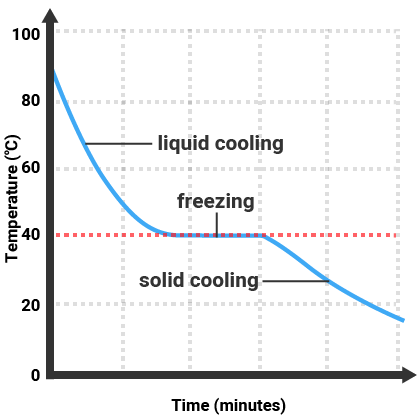 |
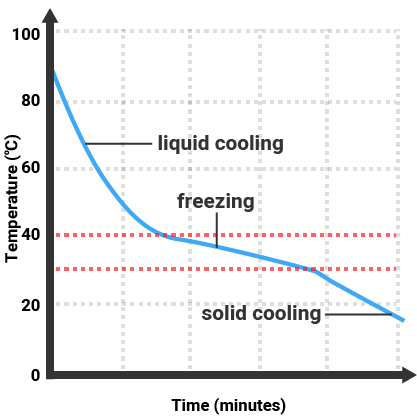 |
| Pure Substance |
Impure Substance |
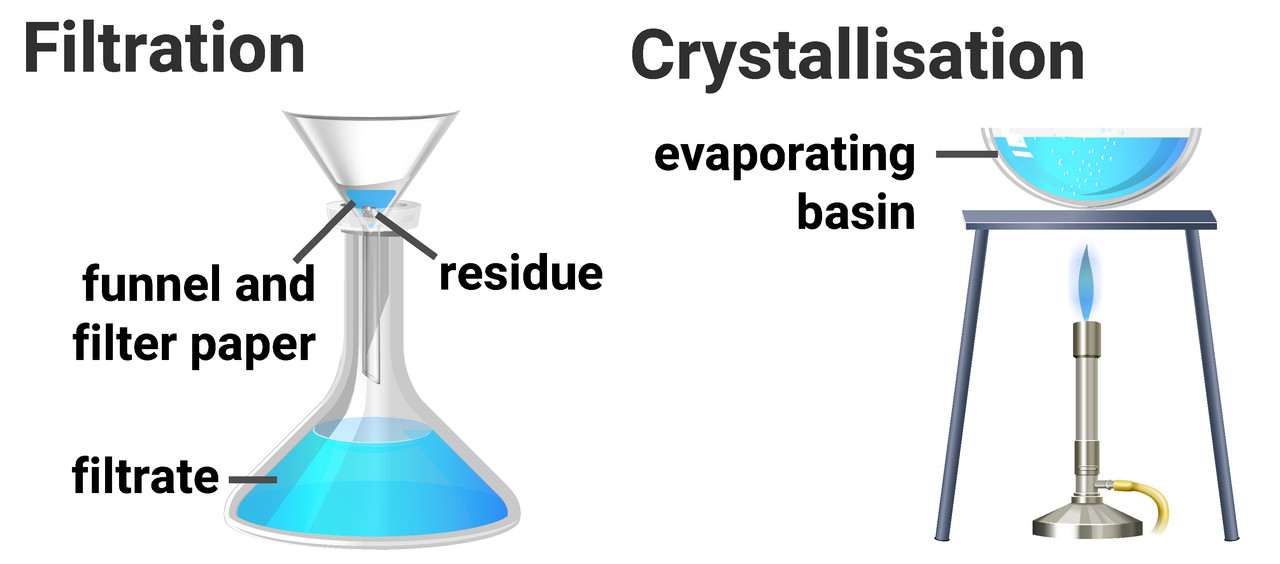
Separation Techniques
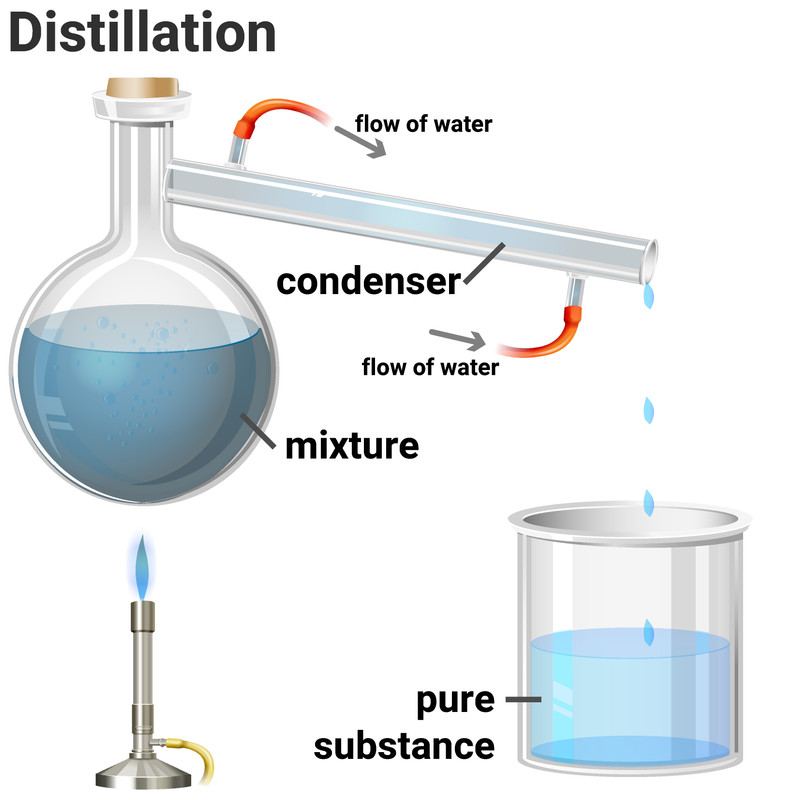
Simple distillation is a method for separating the solvent from a solution, leaving behind the solute. This method works because the solvent has a much lower boiling point than the dissolved solute. Heating the solution allows the solvent to evaporate, and then it passes through a condenser, where it is cooled and condensed into a separate container. The solute does not evaporate and so it stays behind.
Fractional distillation is a method of separating multiple liquids from each other. The technique works in the same way as distillation, but on a much larger scale.
Filtration is a method for separating an insoluble solid from a liquid. A mixture of liquid and solid is passed through filter paper into a flask below. The solid stays on the filter paper (residue), and the liquid passes through to the container below (filtrate).
Crystallisation/evaporation is a method that separates a soluble solid from a liquid. A solution of liquid and dissolved solid is heated, causing the solvent to evaporate and leaves solid crystals behind.
Chromatography
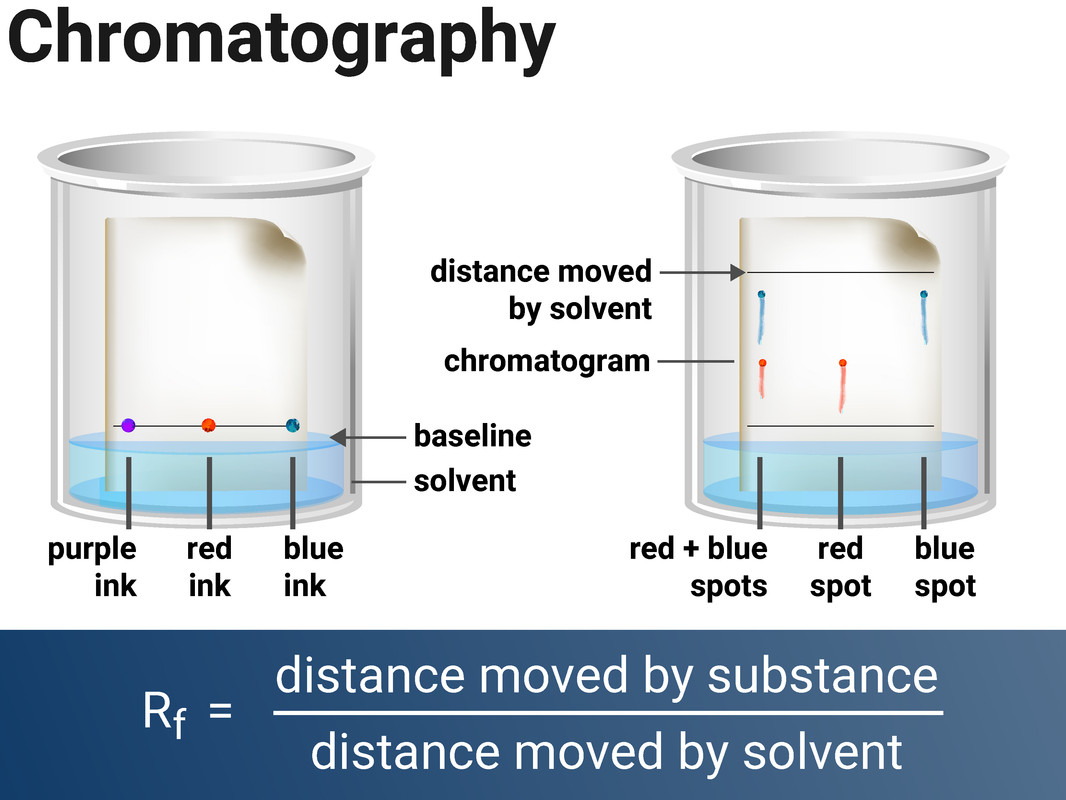
Chromatography is a method for separating dissolved substances from one another. It works because some of the coloured substances dissolve in the solvent better than others, so they travel further up the paper. This technique is often used to separate out inks, or food colourings.
Paper chromatography consists of two stages:
- the stationary phase (a.k.a. the phase that doesn't move - this is the paper)
- the mobile phase (a.k.a. the phase that moves - this is the solvent)
Separation by chromatography produces a chromatogram, and can be used to distinguish between a pure substance (that produces one spot), and a mixture (impure) which produces multiple spots. Different substances will move at different rates through the paper.
When setting up the experiment you must make sure to draw the baseline in pencil, otherwise the ink from a pen would also dissolve in the solvent.
The Rf value of a spot can be used to identify unknown chemicals if they can be compared to a range of reference substances. The Rf value will always be the same for each substance (when the same solvent is used), and an Rf value will always have a value of less than 1. It can be calculated by using:
Rf value = distance moved by substance ÷ distance moved by solvent
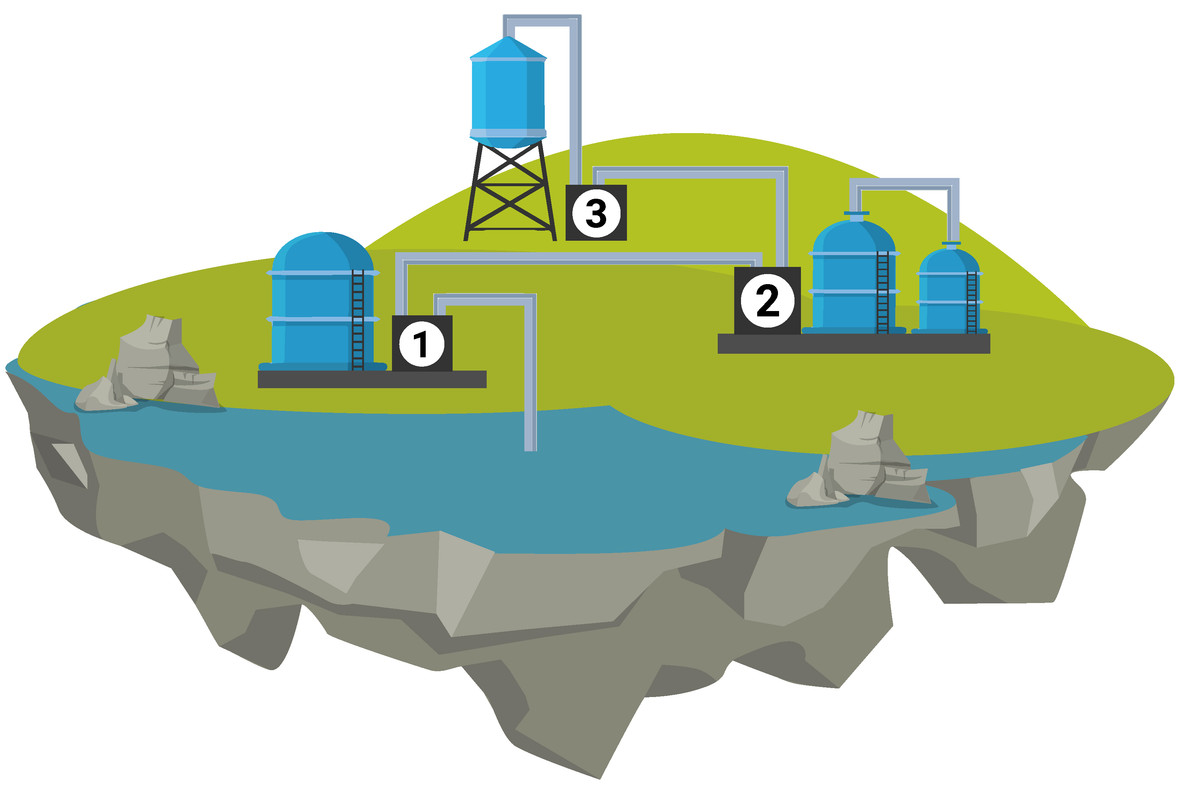
Potable Water
All life requires water to survive. The water they need has to be of good enough quality, which for humans means it needs low levels of dissolved salts and microbes. Water that is safe to drink is called potable water. Potable water is not pure water, because it contains dissolved substances.
There are many methods used to produce potable water, and which method is used will depend on the available supplies of water and local conditions.
In the United Kingdom (UK), rain provides water with low levels of dissolved substances (fresh water) that collects in the ground and in lakes and rivers. To make this water potable, it must go through these steps:
- sedimentation - solids sink to the bottom and are removed
- filtration - fine particles like sand are removed
- chlorination - microbes are killed using chlorine
If supplies of fresh water are limited (such as on an small island), desalination of sea water (high salt levels) may be required. Desalination can be done by distillation. This process requires large amounts of energy and is costly to maintain.
When we do analysis in the lab, we normally use distilled (often called deionised) water. This is because tap water contains dissolved salts that may interfer in our readings, so we must use pure water as these salts have been removed.