Groups in the Periodic Table
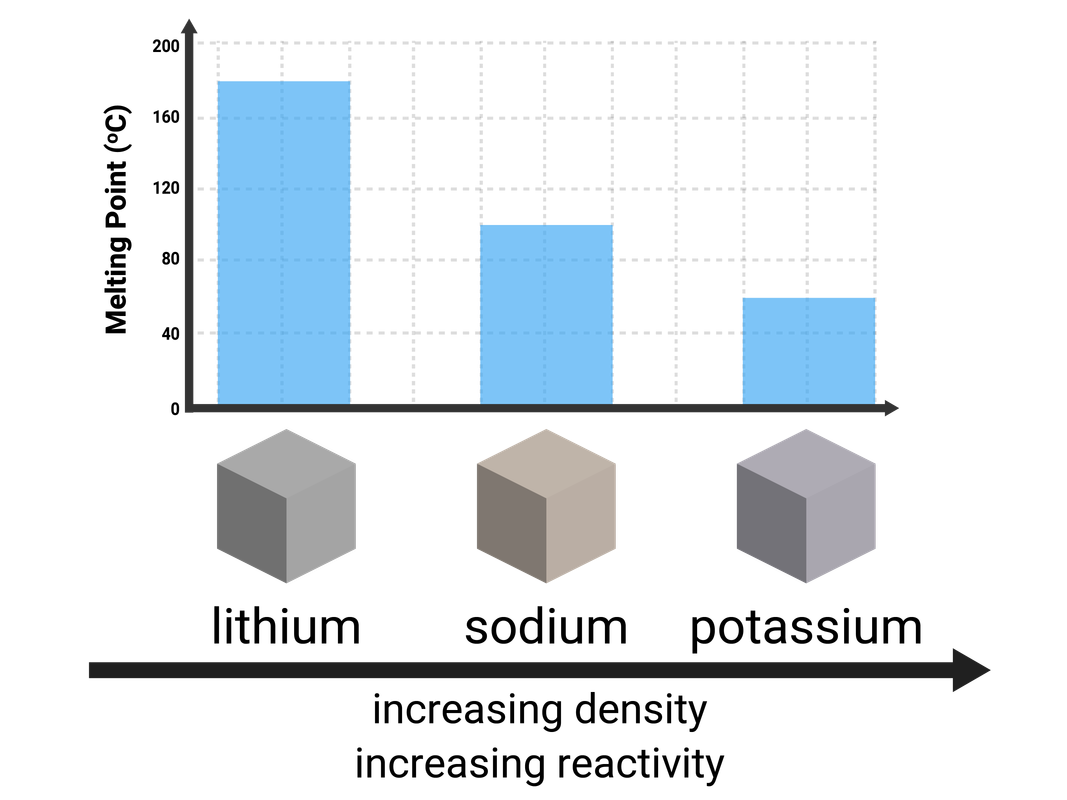
Group 1 (Alkali Metals)
Lithium, sodium, potassium, rubidium, and caesium are all found in Group 1, and are commonly known as the Alkali Metals. Every atom of these elements contains 1 electron on their outermost shell.
These metals are unusual as they:
- are very soft (can be cut with a knife)
- have relatively low melting points, that decrease down the group
These metals have to be stored in oil, as they oxidise in air very easily. We can see this by cutting into the metal: it is shiny on the inside, but dull on the outside.
Group 1 metals will react with water to form an alkaline solution:
- lithium + water → lithium hydroxide + hydrogen
- sodium + water → sodium hydroxide + hydrogen
- potassium + water → potassium hydroxide + hydrogen
Alkali metals become more reactive, the larger the atom. This means the metals near the top of the group (lithium, sodium etc) are much less reactive than those near the bottom of the group (rubidium, caesium etc). This is because the outer negative electron is further away from the positive nucleus, meaning it doesn't feel as much attractive force and so can be more easily lost.
Group 7 (Halogens)
Fluorine, chlorine, bromine, and iodine are found in Group 7, and are commonly known as the Halogens. Every atom of these elements contains 7 electrons on their outermost shell.
At room temperature these elements in various states, and have very different colours:
| chlorine, Cl2 | bromine, Br2 |
iodine, I2 |
|---|---|---|
| pale green gas | brown liquid | purple-black solid |
Notice that moving from the top of the group (fluorine - gas) to the bottom of the group (astatine - solid), shows the melting/boiling points of these elements increases as the states they are found in at room temperature change quite significantly.
This is because:
- the molecules become larger
- the intermolecular forces between molecules become stronger
- and so more energy is needed to overcome these forces
Reactions of the Halogens
Group 7 elements will react with metals to form metal halides:
- chlorine + iron → iron fluoride
- bromine + sodium → sodium bromide
- iodine + magnesium → magnesium iodide
Group 7 elements will react with hydrogen to form hydrogen halides (which dissolve in water to form acidic solutions):
- chlorine + hydrogen → hydrogen chloride
- bromine + hydrogen → hydrogen bromide
- iodine + hydrogen → hydrogen iodide
The Group 7 elements become less reactive, the larger the atom. This means the elements near the top of the group (fluorine, chlorine etc) are much more reactive than those near the bottom of the group (iodine, astatine etc). This is because the outer negative electron shell is further away from the positive nucleus, meaning it doesn't feel as much attractive force and so is more difficult to attract an electron.
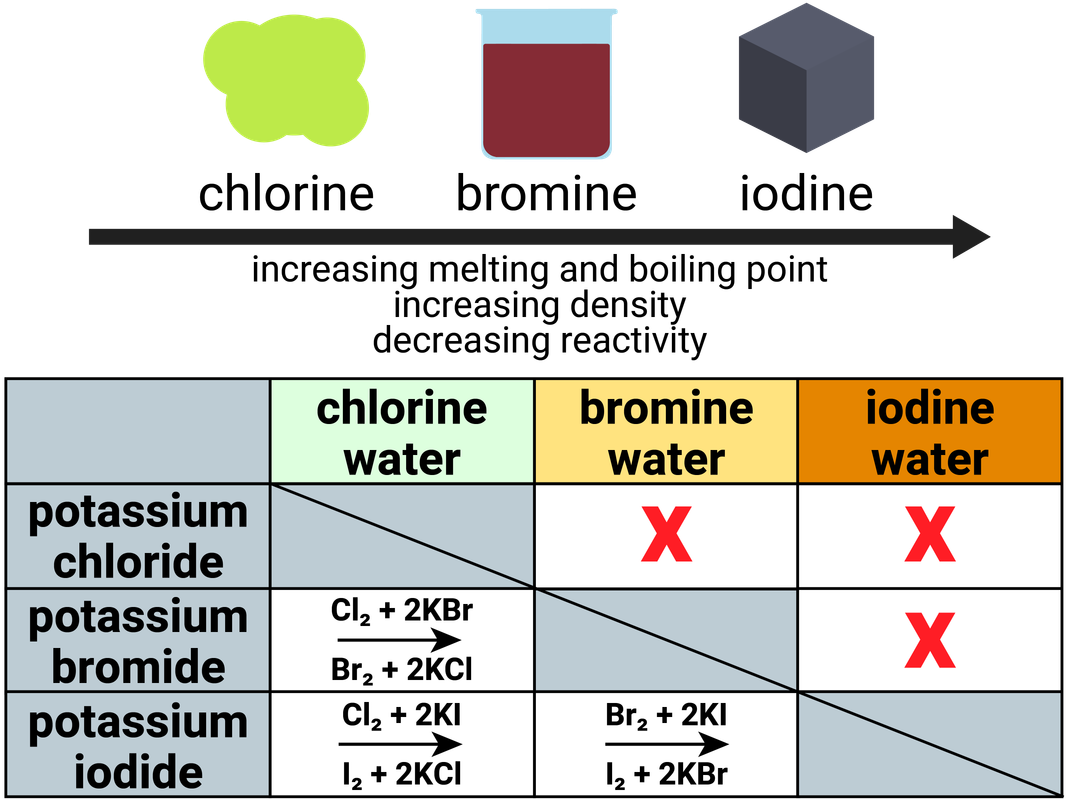
Displacement reactions
A more reactive halogen will displace a less reactive halogen in solution:
- chlorine + sodium bromide → bromine + sodium chloride
- chlorine + sodium iodide → iodine + sodium chloride
- bromine + sodium iodide → iodine + sodium bromide
Higher Tier
Displacement reactions are a type of redox chemistry, i.e. there are oxidation and reduction reactions happening. We can see this when we split up a halogen displacement equation into half equations:
chlorine + sodium bromide → sodium chloride + bromine
Cl2 + 2NaBr → 2NaCl + Br2
2Br- → Br2 + 2e-
Cl2 + 2e- → 2Cl-
In this example, bromine is oxidised (loss of electrons) and the chlorine is reduced (gain of electrons).
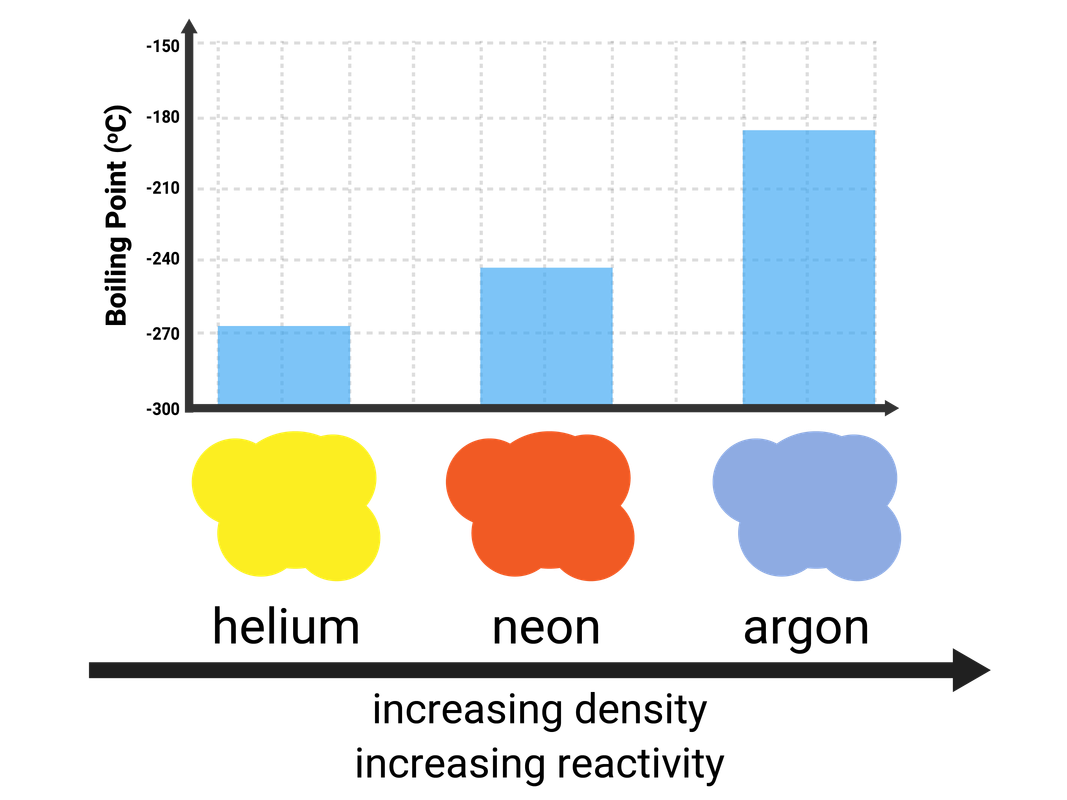
Group 0 (Noble Gases)
Helium, neon, argon, krypton, and xenon are all Noble Gases, because they have full outershells of electrons and are stable. This means they are unreactive, as they do not need to react to gain electrons - so they are called inert.
These gases are all colourless.
All of the elements in this group are gases at room temperature, as they have very low melting and boiling points. The boiling points do increase down the group, and this is due to stronger intermolecular forces of attraction (meaning more energy is needed to break them).
The noble gases have many uses, mainly due to them being so unreactive (and therefore non-flammable) and their low density. Some examples:
- helium is used in balloons (due to low density and being non-flammable)
- argon is used as a 'shield gas' when welding pieces of metal together (denser than air, and stops the metal oxidising)
- when electricity is passed through these gases they glow different colours:
- helium glows yellow
- neon glows orange-red
- argon glows blue-lilac