
Heat Energy Changes in Chemical Reactions
Rates of Reaction and Energy Changes
Energy
Chemicals store energy in their bonds. If the products of a reaction store more energy than the reactants, then energy must have been taken in from the surroundings. The overall amount of energy, however, doesn't change (if this is a closed system) - we know this is true, because energy cannot be created or destroyed.
When reactions happen in solution, if there are temperature changes that can be observed they can be measured using a thermometer.
Reactions that take in more heat energy from their surroundings (than they give out) are called endothermic reactions. Examples include:
- electrolysis
- photosynthesis
- thermal decomposition
Reactions that release more heat energy to their surroundings (than they take in) are called exothermic reactions. Examples include:
- combustion
- neutralisation
- displacements
Depending on the reactants, the following reactions can be either exothermic or endothermic:
- salts dissolving in water
- precipitation reactions
Chemical reactions can be thought of as happening in two stages:
- Energy is needed to break the bonds in the reactants to form their separate atoms. This energy is called the activation energy, and is an endothermic process.
- The newly separated atoms form new bonds to make the products, and release energy to their surroundings. This is an exothermic process.
The overall net movement of energy determines if the reaction is endothermic or exothermic. The reaction is:
- exothermic if more heat energy is released in forming bonds in the products than is required in breaking bonds in the reactants
- endothermic if less heat energy is released in forming bonds in the products than is required in breaking bonds in the reactants
Reaction Profiles
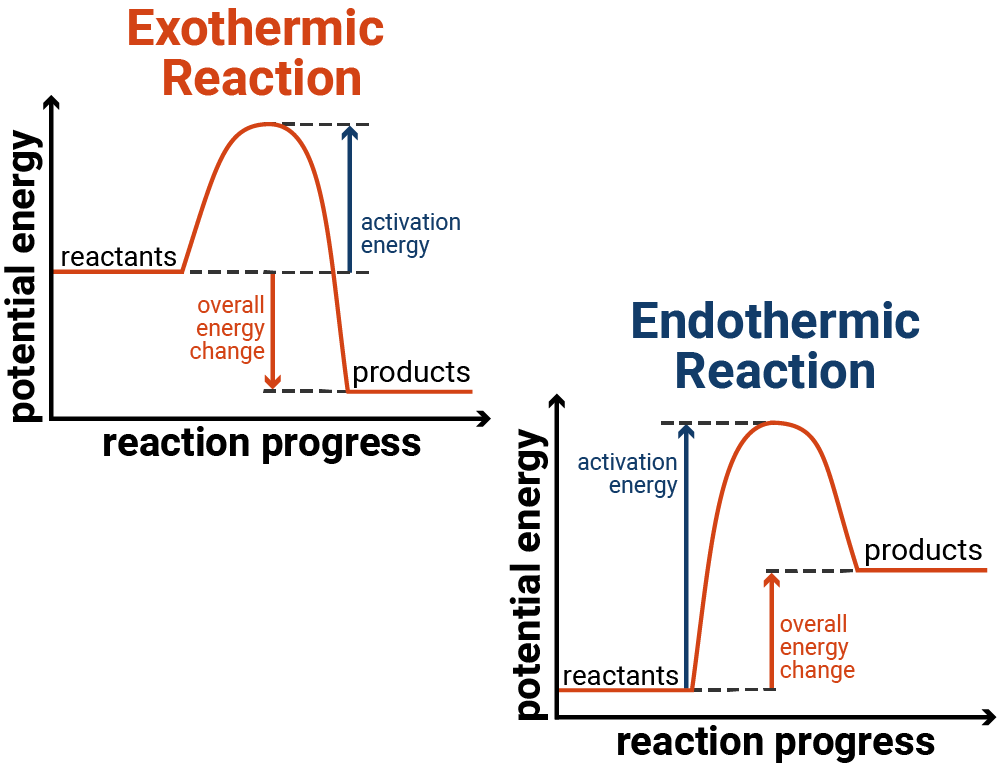
These are diagrams that show the relative energies of the reactants and products in a reaction - to show how energy changes over the course of a reaction.
All reaction profiles start with an initial rise in energy, this is the activation energy and is the energy required for the reaction to begin. Whether the reaction then releases more (or less) energy than it initially required will determine if it is an exothermic or endothermic reaction.
Exothermic Reactions
A reaction that releases energy can be shown on a reaction profile to initially have an increase (due to breaking bonds of reactants), but then has a bigger drop in energy overall (due to this energy being released from making new bonds).
Endothermic Reactions
A reaction that takes in energy can be shown on a reaction profile to initially have an increase (due to breaking bonds of reactants), but then has a smaller drop in energy (due to bonds being made).
Bond Energies
Higher Tier
Every chemical bond has a specific bond energy. Whilst the energies vary slightly depending on the compound, we can assume this difference won't affect our calculations.
A bond energy tells you how much energy is required to break the bond. If more energy is required to break reactant bonds - the reaction is endothermic. If more energy is released by making product bonds - the reaction is exothermic.
Some common bond energies are given in the table below.
| Bond | Average Bond Energy (kJ/mol) |
|---|---|
| C-C | 347 |
| C=C | 602 |
| C=O | 799 |
| C-H | 413 |
| H-O | 464 |
| Cl-Cl | 243 |
| H-Cl | 432 |
To work out the overall energy change in a reaction:
- calculate the total energy in the reactants
- calculate the total energy in the products
- subtract the energy in the products from the energy from the reactants
- if you have a positive (+) value, then your energy change is endothermic; if you have a negative (-) value, then your change is exothermic.
Hydrogen and chlorine react to form hydrogen chloride gas:
H2 + Cl2 → 2HCl
H-H + Cl-Cl → 2 × H-Cl
Energy in = 436 + 243 = 679 kJ mol-1
Energy out = 2 × 432 = 864 kJ mol-1
Energy change = in - out
Energy change = 679 - 864 = -185 kJ mol-1
As the energy change is negative this shows that the reaction is exothermic, because the 'energy out' through making bonds is larger than the 'energy in' to break bonds