Predicting chemical reactions
C4: Predicting and identifying reactions and products
Group 1 (Alkali Metals)
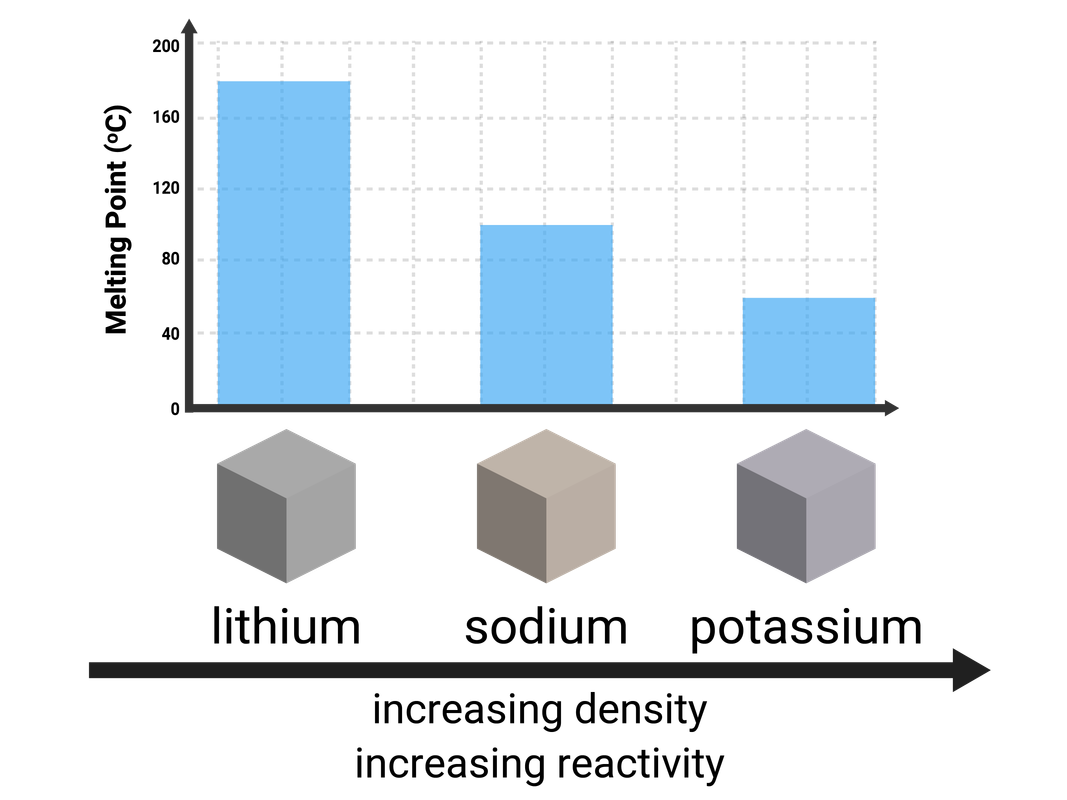
Lithium, sodium, potassium, rubidium, and caesium are all found in Group 1, and are commonly known as the Alkali Metals. Every atom of these elements contains 1 electron on their outermost shell.
These metals are unusual as they:
- are very soft (can be cut with a knife)
- have relatively low melting points, that decrease down the group
These metals have to be stored in oil, as they oxidise in air very easily. We can see this by cutting into the metal: it is shiny on the inside, but dull on the outside.
Group 1 metals will react with water to form an alkaline solution:
- lithium + water → lithium hydroxide + hydrogen
- sodium + water → sodium hydroxide + hydrogen
- potassium + water → potassium hydroxide + hydrogen
Alkali metals become more reactive, the larger the atom. This means the metals near the top of the group (lithium, sodium etc) are much less reactive than those near the bottom of the group (rubidium, caesium etc). This is because the outer negative electron is further away from the positive nucleus, meaning it doesn't feel as much attractive force and so can be more easily lost.
Group 7 (Halogens)
Fluorine, chlorine, bromine, and iodine are found in Group 7, and are commonly known as the Halogens. Every atom of these elements contains 7 electrons on their outermost shell.
At room temperature these elements in various states, and have very different colours:
| chlorine, Cl2 | bromine, Br2 |
iodine, I2 |
|---|---|---|
| pale green gas | brown liquid | purple-black solid |
Notice that moving from the top of the group (fluorine - gas) to the bottom of the group (astatine - solid), shows the melting/boiling points of these elements increases as the states they are found in at room temperature change quite significantly.
This is because:
- the molecules become larger
- the intermolecular forces between molecules become stronger
- and so more energy is needed to overcome these forces
Reactions of the Halogens
Group 7 elements will react with metals to form metal halides:
- chlorine + iron → iron fluoride
- bromine + sodium → sodium bromide
- iodine + magnesium → magnesium iodide
Group 7 elements will react with hydrogen to form hydrogen halides (which dissolve in water to form acidic solutions):
- chlorine + hydrogen → hydrogen chloride
- bromine + hydrogen → hydrogen bromide
- iodine + hydrogen → hydrogen iodide
The Group 7 elements become less reactive, the larger the atom. This means the elements near the top of the group (fluorine, chlorine etc) are much more reactive than those near the bottom of the group (iodine, astatine etc). This is because the outer negative electron shell is further away from the positive nucleus, meaning it doesn't feel as much attractive force and so is more difficult to attract an electron.
Displacement reactions
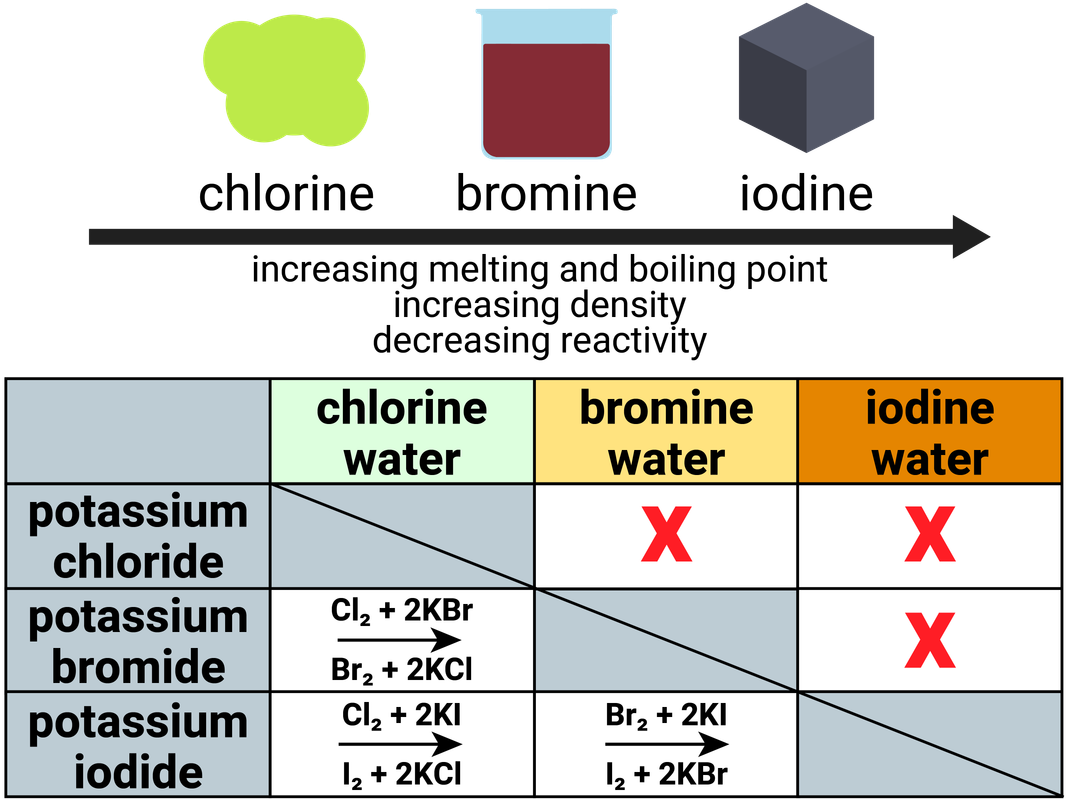
A more reactive halogen will displace a less reactive halogen in solution:
- chlorine + sodium bromide → bromine + sodium chloride
- chlorine + sodium iodide → iodine + sodium chloride
- bromine + sodium iodide → iodine + sodium bromide
Higher Tier
Displacement reactions are a type of redox chemistry, i.e. there are oxidation and reduction reactions happening. We can see this when we split up a halogen displacement equation into half equations:
chlorine + sodium bromide → sodium chloride + bromine
Cl2 + 2NaBr → 2NaCl + Br2
2Br- → Br2 + 2e-
Cl2 + 2e- → 2Cl-
In this example, bromine is oxidised (loss of electrons) and the chlorine is reduced (gain of electrons).
Group 0 (Noble Gases)
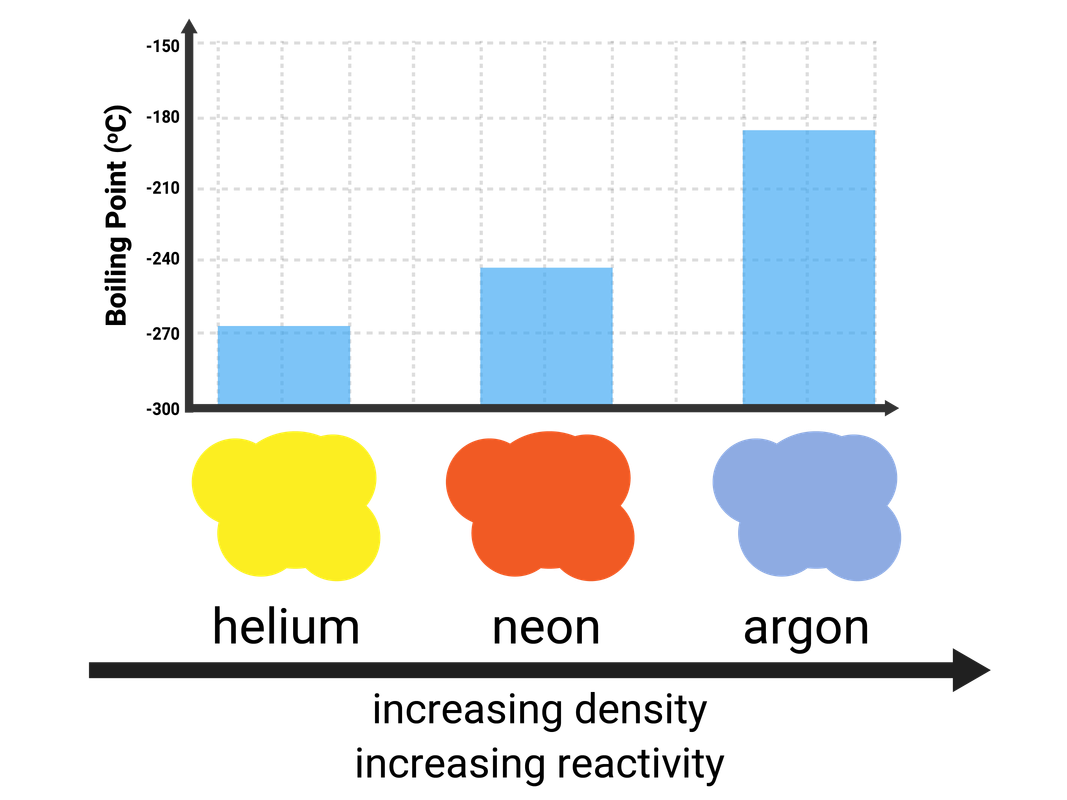
Helium, neon, argon, krypton, and xenon are all Noble Gases, because they have full outershells of electrons and are stable. This means they are unreactive, as they do not need to react to gain electrons - so they are called inert.
The noble gases have eight electrons in their outer shell, except for helium, which has
only two electrons.
These gases are all colourless.
All of the elements in this group are gases at room temperature, as they have very low melting and boiling points. The boiling points do increase down the group, and this is due to stronger intermolecular forces of attraction (meaning more energy is needed to break them).
The noble gases have many uses, mainly due to them being so unreactive (and therefore non-flammable) and their low density. Some examples:
- helium is used in balloons (due to low density and being non-flammable)
- argon is used as a 'shield gas' when welding pieces of metal together (denser than air, and stops the metal oxidising)
- when electricity is passed through these gases they glow different colours:
- helium glows yellow
- neon glows orange-red
- argon glows blue-lilac
Reactivity Series
When metals react with other substances the metal atoms form positive ions. The reactivity of a metal is related to its tendency (how likely it is) to form positive ions.
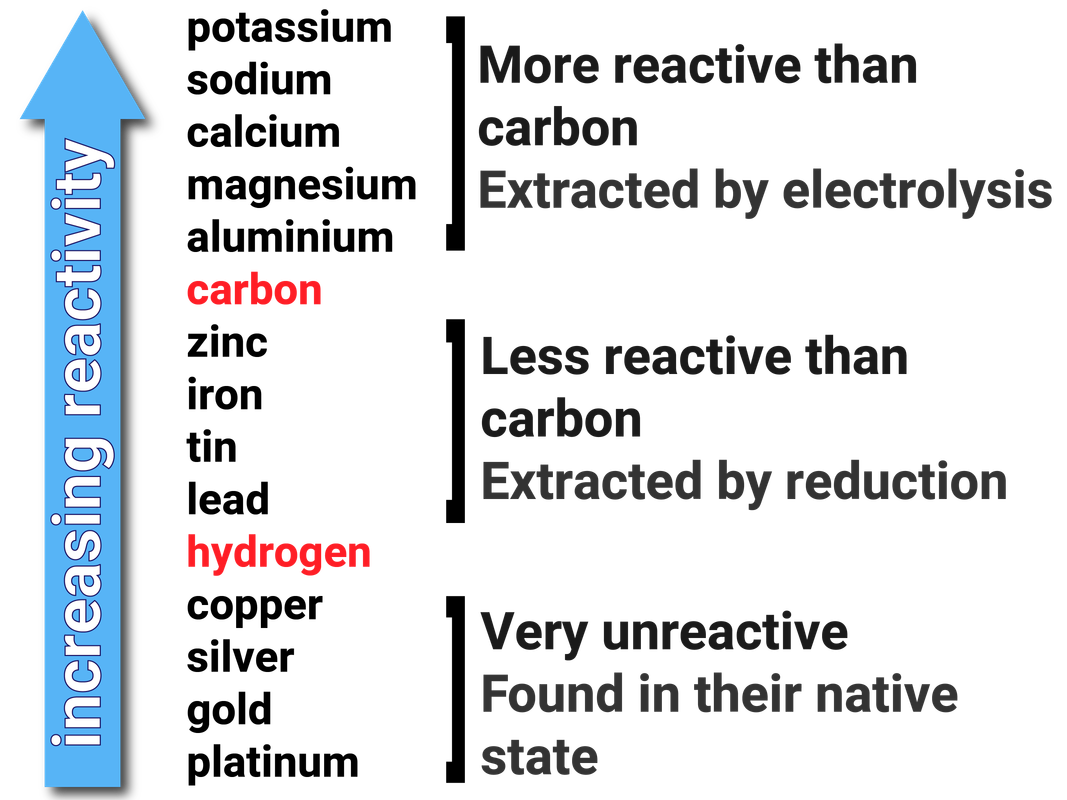
Metals can be arranged in order of their reactivity in a reactivity series. The metals potassium, sodium, lithium, calcium, magnesium, zinc, iron and copper can be put in order of their reactivity from their reactions with water and dilute acids.
The non-metals hydrogen and carbon are often included in the reactivity series to give an indication about how the metals can be extracted.
Metals less reactive than hydrogen are generally found in their elemental form in the Earth's crust (native state). They do not need to be extracted.
Metals less reactive than carbon can be extracted by heating the metal with carbon. As the carbon is more reactive, it will displace the metal in its ore - reducing the metal.
Metals more reactive than carbon have to be extracted using electrolysis. This is very costly, in terms of both money and energy.
Reactions of Metals
All metals will react with substances differently, and even the same metal can be more or less reactive depending on what it is being reacted with.
Rather than having multiple tables for each specific reaction, a diagram was created to show the average reactivity of metals with water and dilute acids.
The reactivity series can also be used to see the relative tendency for metal atoms to form cations - the higher up the series, the more likely it will form a positive ion.
Something similar can be said for a metal's resistance to oxidation - the higher up the series, the more easier it is to oxidise.
| metal | reaction with water | reaction with dilute acid |
|---|---|---|
| potassium | react with cold water to form hydrogen and a metal hydroxide | react violently |
| sodium | ||
| calcium | react to form hydrogen and a salt solution | |
| magnesium | react very slowly, if at all, with cold water - but will react with steam to form hydrogen and a metal oxide | |
| aluminium | ||
| zinc | ||
| iron | ||
| copper | react with cold water to form hydrogen and a metal hydroxide | do not react |
| silver | ||
| gold |
Displacement Reactions
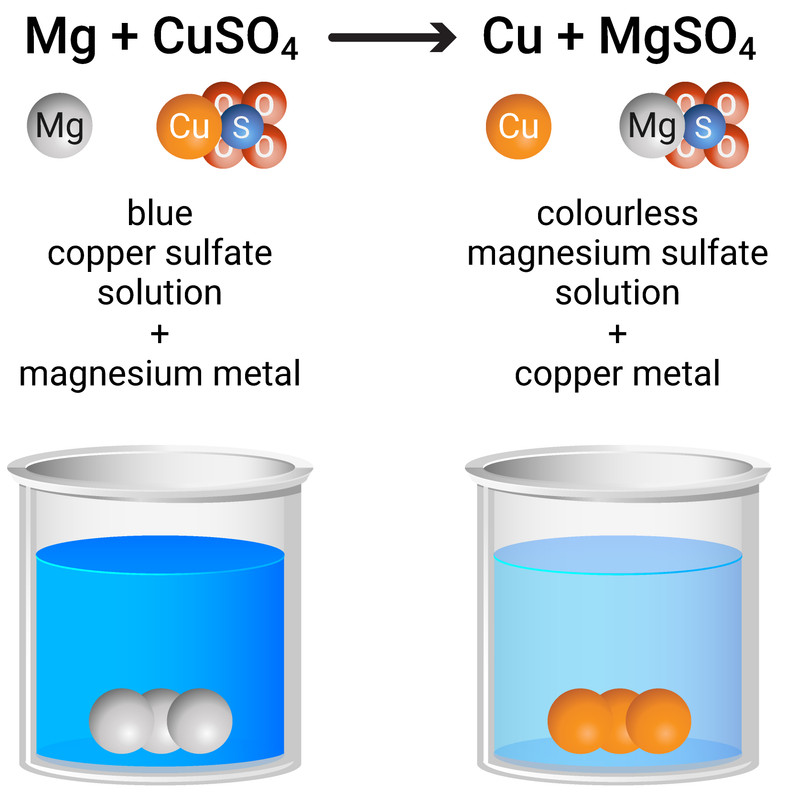
A more reactive metal can displace a less reactive metal from a compound in solution. This is one way to extract metals. For example:
magnesium + copper sulfate → copper + magnesium sulfate
Mg + CuSO4 → Cu + MgSO4
Higher Tier
Ionic equations only show us the ions that change in a chemical reaction. This is useful as it shows what is oxidised and reduced. We can start by writing a balanced symbol equation for a reaction:
Mg(s) + CuSO4(aq) → Cu(s) + MgSO4(aq)
Then we can write out all the ions involved:
Cu²⁺(aq) + SO4²⁻(aq) + Mg(s) → Cu(s) + Mg²⁺(aq) + SO4²⁻(aq)
We can identify the ions that don't change, these are called the spectator ions. These are of no interest in the reaction, so we can ignore them and write out our ionic equation:
Mg(s) + Cu²⁺(aq) → Cu(s) + Mg²⁺(aq)
Once we have an ionic equation, we can take it one step further and look at half equations. These look at how the electrons behave. We can see there are only two different chemicals involved, and we can split our equation in half... to make half equations:
Cu²⁺ + 2e⁻ → Cu
Mg - 2e⁻ → Mg²⁺
By writing our equation in this way, we can immediately see that copper had to gain two electrons (reduced) in this reaction, and those electrons came from magnesium (oxidised).