The Periodic Table
Key Concepts in Chemistry
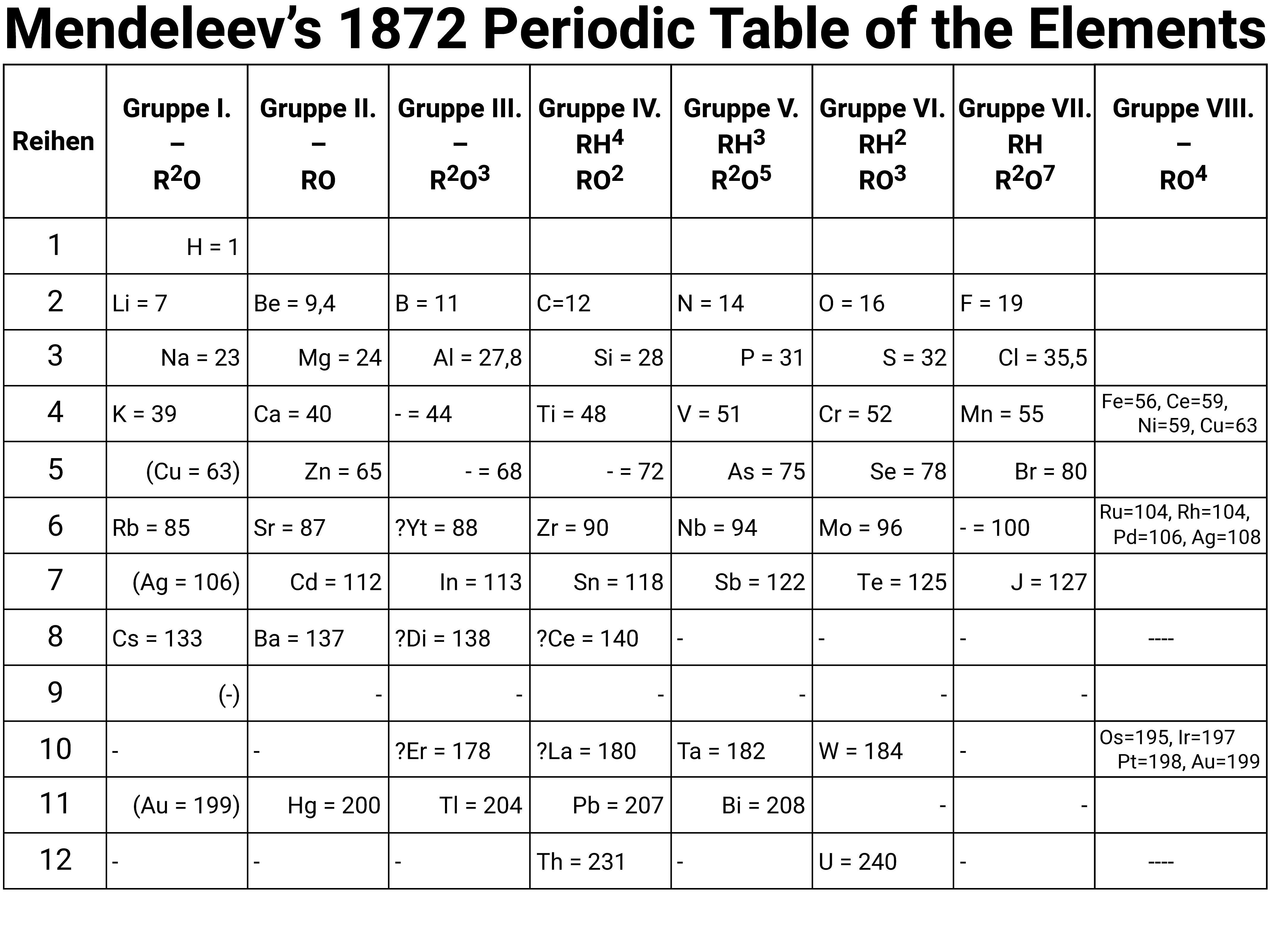
History of the Periodic Table
Mendeleev arranged his table in order of increasing relative atomic mass, as well as using the properties of known elements and compounds.
Trying to order them based on mass was difficult, and sometimes led to untrue positions due to the fact that Mendeleev was unaware at the time about isotopes and their relative abundancies.
Where elements didn't fit 'the pattern', Mendeleev moved them so that his table made more sense. This often led to there being gaps in the table, and Mendeleev is most famous for leaving these gaps claiming they were due to undiscovered elements. For some of these elements, he predicted their properties and masses... and got them right!
Mendeleev's Table (Key)
The symbols R²O and RH⁴, use superscripts to show the number of atoms in molecules rather than the current style of using subscripts. For group 1 ("Gruppe I") this would mean they could form molecules like H²O, and Li²O ... which we know today is correct.
The gaps marked with hyphens show elements deduced by Mendeleev as existing, but unknown in 1872; he predicted the properties of some of these elements.
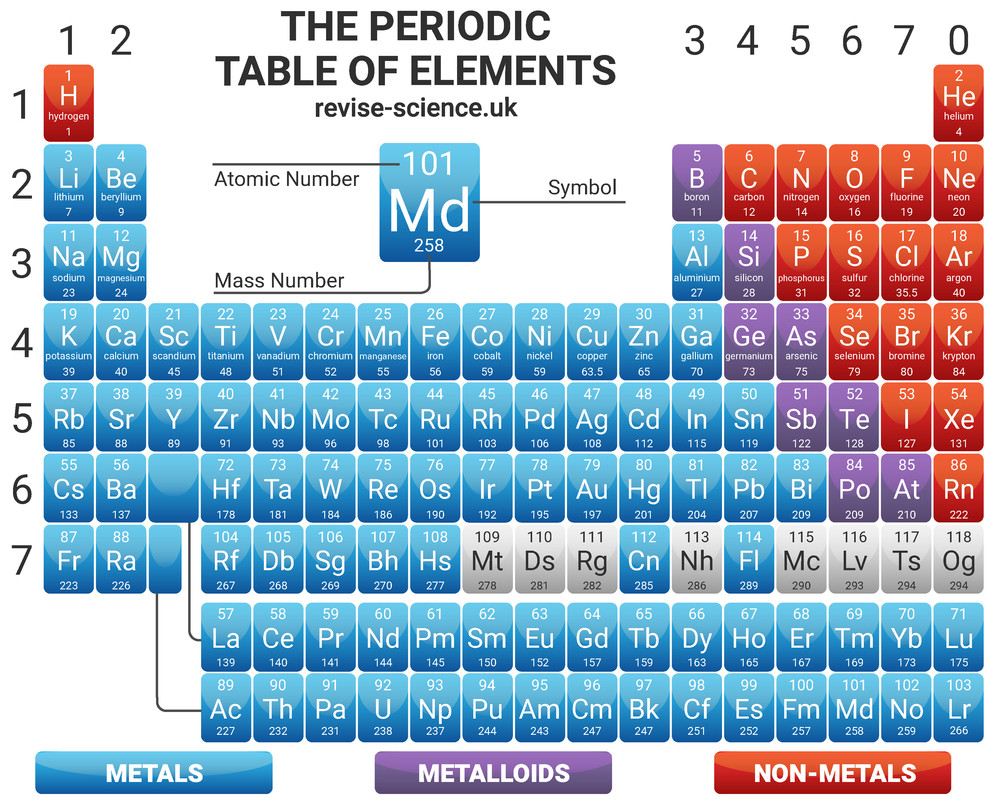
Modern Periodic Table
On the Periodic Table you will find every element that we know about, which in turn is a list of every atom that we have discovered. It is ordered by atomic number, meaning that reading from left to right increases the proton number by one every time.
The Periodic Table is arranged in such a way that elements with similar properties can be found together in columns called 'groups' - for example the group 1 metals are all very reactive with water. Elements in the same group have the same number of electrons in their outershell (these are known as valence electrons).
Elements in the same period have the same number of electron shells.
The Periodic Table is split with metals all found on the left side of the table, and non metals found on the right. Metals have either 1, 2 or 3 electrons on their outermost shell, so want to lose electrons to form positive ions (cations). Non-metals that have either 5, 6 or 7 electrons on their outermost shell want to gain electrons to form negative ions (anions). Non-metals with 8 electrons, the Noble Gases, don't lose or gain electrons to form ions, as they are stable (and therefore inert).