Acids and Salts
Chemical Changes
pH and Neutralisation
When universal indicator (UI) is added to solutions, it will change colour depending on what ions are present. UI can be used to estimate the pH of a substance using a colour chart, however pH probes give a much more accurate reading of pH.
The coloured pH scale below can only be used with indicators like UI, because these are the colours UI turns. Other indicators like methyl orange, and phenolphthalein, do not turn these colours - so this scale cannot be used.
| pH 1 | pH 2 |
pH 3 | pH 4 | pH 5 | pH 6 | pH 7 |
pH 8 | pH 9 | pH 10 | pH 11 | pH 12 | pH 13 | pH 14 | ||||||||||||||
| strong acid | acid | weak acid | neutral | weak alkali | alkali | strong alkali | |||||||||||||||||||||
Acids are made up of hydrogen ions (H+), which are released when acids are dissolved in water. The more hydrogen ions there are in solution, the lower the pH of that solution.
A base is a substance that can neutralise an acid to form a salt and water only. The most common bases you will come across are metal oxides and metal hydroxides. Metal carbonates are not quite bases as they also produce carbon dioxide gas. Most bases are insoluble in water, and those that are soluble are called alkalis. Alkalis are made up of hydroxide ions (OH-). When an acid and an alkali react, we can write an ionic equation to show how they make water:
H+(aq) + OH-(aq) → H2O(l)
When a base and an acid react, they produce a substance that is neutral. We call this a neutralisation reaction. A salt and water are always made during a neutralisation reaction.
acid + base → salt + water
Indicators
Indicators tells us what the pH is of a chemical by changing colour. Different indicators turn different colours based on what type of chemical is present.
| litmus | methyl orange | phenolphthalein | |
|---|---|---|---|
| acid | red | red | colourless |
| neutral | purple | orange | colourless |
| alkali | blue | yellow | pink/purple |
Higher Tier
The higher the concentration of hydrogen ions in an acidic solution, the lower the pH.
The higher the concentration of hydroxide ions in an alkaline solution, the higher the pH.
As hydrogen ion concentration in a solution increases by a factor of 10, the pH of the solution will decrease by 1.
Dilute/Concentrated and Weak/Strong
Higher Tier
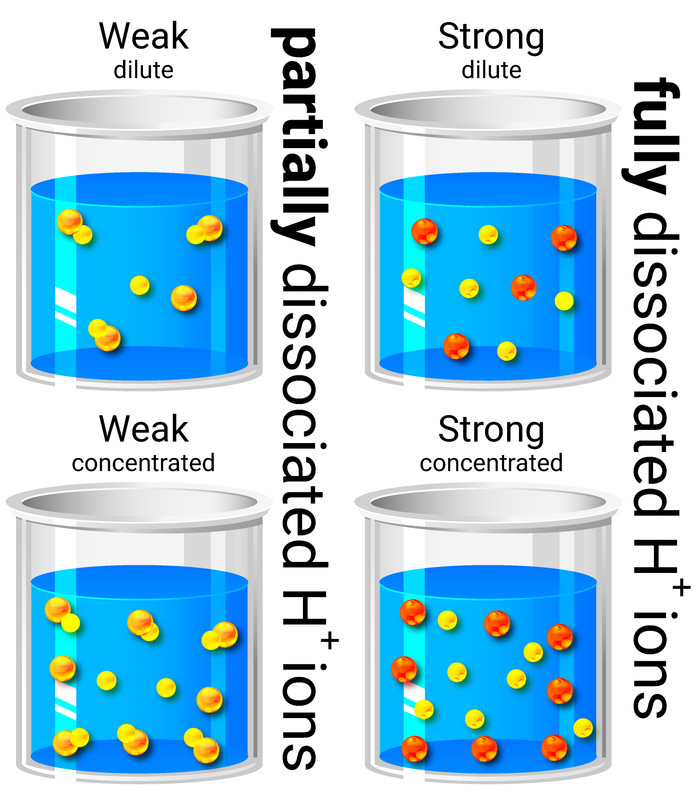
A solution forms when a solute dissolves in a solvent. The more concentrated the solution, the more particles it contains in a given volume.
When solutions are described as dilute or concentrated:
- a dilute solution contains a small amount of dissolved solute
- a concentrated solution contains a large amount of dissolved solute
Acids in solution are a source of hydrogen ions (H+). The hydrogen ions are produced when the acid dissociates (splits up) to form ions.
When acids are described as weak or strong:
- weak acids only partially dissociate into ions in solution, for example ethanoic acid
- strong acids completely dissociate into ions in solution, for example hydrochloric acid
General Reactions
When acids react with different chemicals, they can make different products. Using different acids will create salts that have different names. In the table below you can see how each of these acids affects the name of the possible salt:
| hydrochloric acid | sulfuric acid | nitric acid | |
|---|---|---|---|
| salt name | metal chloride | metal sulfate | metal nitrate |
Acids + Metals
A salt and hydrogen are produced when acids react with metals.
- acid + metal → salt + hydrogen
- hydrochloric acid + magnesium → magnesium chloride + hydrogen
- 2HCl(aq) + Mg(s) → MgCl2(aq) + H2(g)
Acids + Metal Oxides
A salt and water are produced when acids react with metal oxides.
- acid + metal oxide → salt + water
- nitric acid + lithium oxide → lithium nitrate + water
- 2HNO3(aq) + Li2O(s) → 2LiNO3(aq) + H2O(l)
Acids + Metal Hydroxides
A salt and water are produced when acids react with metal hydroxides.
- acid + metal hydroxide → salt + water
- sulfuric acid + sodium hydroxide → sodium sulfate + water
- H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + 2H2O(l)
Acids + Metal Carbonates
A salt, water, and carbon dioxide are produced when acids react with metal carbonates.
- acid + metal hydroxide → salt + water + carbon dioxide
- sulfuric acid + calcium carbonate → calcium sulfate + water + carbon dioxide
- H2SO4(aq) + CaCO3(s) → CaSO4(aq) + H2O(l) + CO2(g)
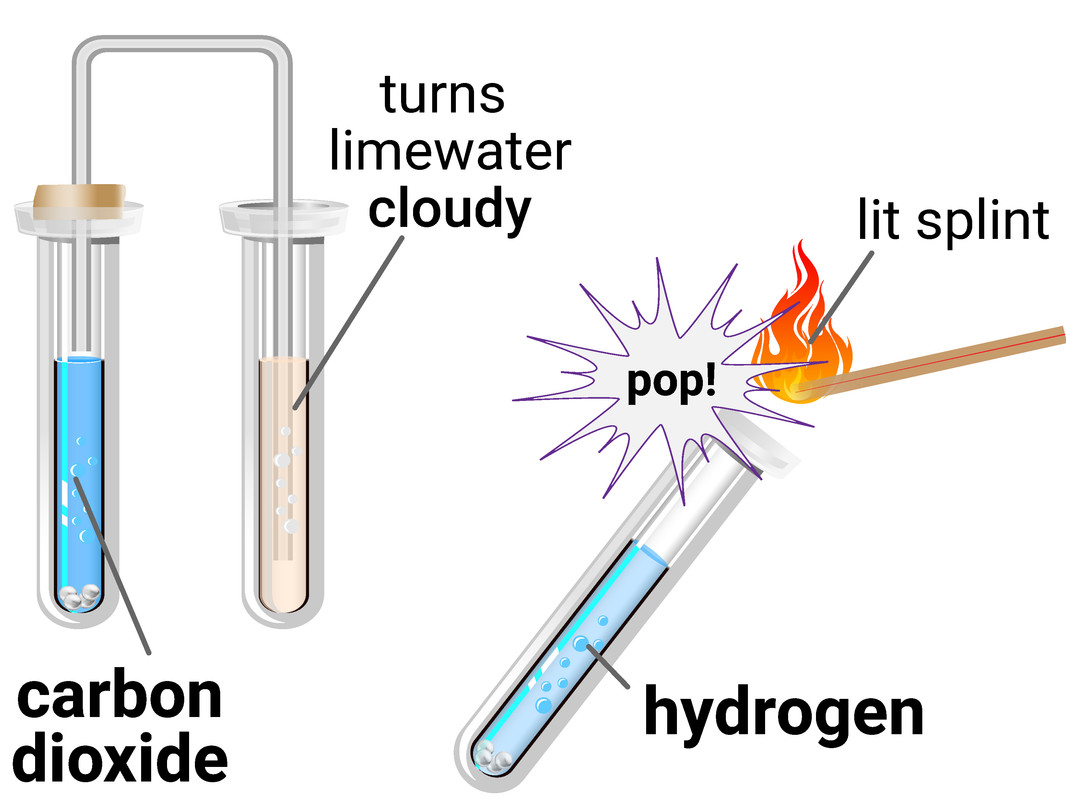
Test for Gases
Hydrogen
The test for hydrogen uses a lit (burning) splint held at the end of a container of the gas. If you hear a 'squeaky pop' then hydrogen is present.
This test requires you to allow a second, or two, for oxygen gas from the air to mix with the hydrogen first, as the test is actually showing the chemical reaction between hydrogen and oxygen to make water.
Carbon Dioxide
The test for carbon dioxide involves bubbling the gas through limewater (calcium hydroxide solution). If the limewater turns milky/cloudy then carbon dioxide is present.
This test is actually a reaction between the carbon dioxide and limewater, to make limestone (calcium carbonate).
Making Soluble Salts
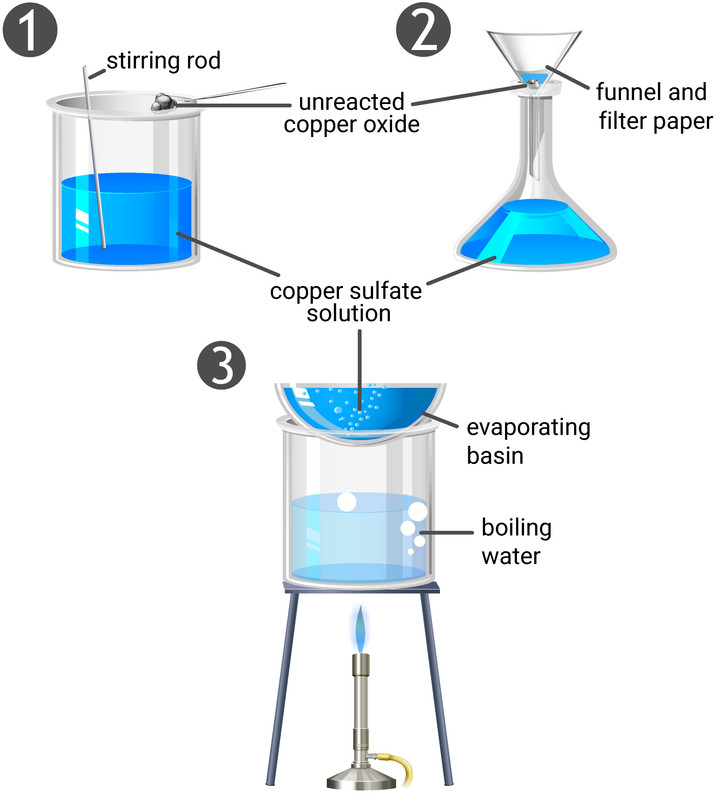
From acids and insoluble reactants
- excess of the reactant (a metal, metal oxide, or metal carbonate) is added
- excess is added to make sure all of the acid reacts, and is gently heated to speed up the reaction
- the excess reactant has to be removed
- because we added more reactant than we need, we have to remove any left over by filtration
- the solution remaining is only salt and water
- the acid is used up and the insoluble reactant has been removed
- evaporating the water leaves the pure, dry crystals of salt
- the acid is used up and the insoluble reactant has been removed
From acids and soluble reactants
- a titration must be used
- this is because there is no insoluble excess reactant that we can remove using filtration
- the acid and the soluble reactant (usually a dilute alkali) are mixed in the correct proportions
- because we want to exactly neutralise the acid, adding any less, or more, alkali than needed will not result in a neutral solution of salt
- the solution remaining is only salt and water
- the reactant has been added in correct proportions to fully react with the acid
- evaporating the water leaves the pure, dry crystals of salt
- the reactant has been added in correct proportions to fully react with the acid
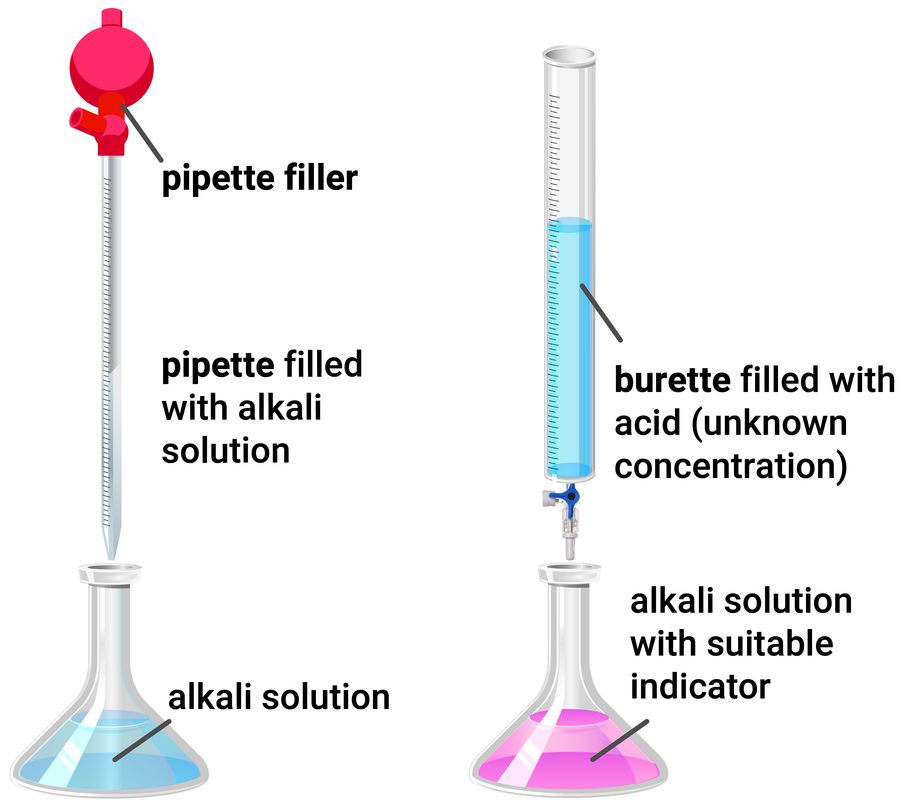
Titrations
Apparatus
- A glass pipette is used to accurately measure the volume of a reactant before transferring it to a conical flask
- A burette is used to add small, exact volumes of one reactant to the other reactant
- A suitable indicator, usually either methyl orange or phenolpthalein (as these give exact colour changes, rather than UI that has too many colours and is difficult to tell when neutral)
Method
- Using the pipette and pipette filler, add 25 ml of alkali to a clean conical flask
- Add a few drops of suitable indicator to the alkali, and put the conical flask on a white tile
- Fill the burette with acid and record the starting volume
- Slowly add the acid from the burette to the conical flask, swirling it to make sure they mix
- Stop adding the acid when the end-point is reached (this is when the indicator permanently changes colour), and record the final volume reading
- Repeat steps 1 to 5 until you get concordant titres. You will achieve more accurate results if acid is added drop by drop near to the end-point (you will be able to calculate this from your rough titre
Results and analysis
Readings should be recorded to two decimal places (where the final digit is either a 0 or 5).
You need to work out a mean titre, and you must only include results that are concordant in your calculation.
| rough | run 1 | run 2 | run 3 | |
|---|---|---|---|---|
| final reading | 26.55 | 26.35 | 26.80 | 26.25 |
| initial reading | 0.00 | 0.15 | 0.50 | 0.00 |
| titre (ml) | 26.55 | 26.20 | 26.30 | 26.25 |
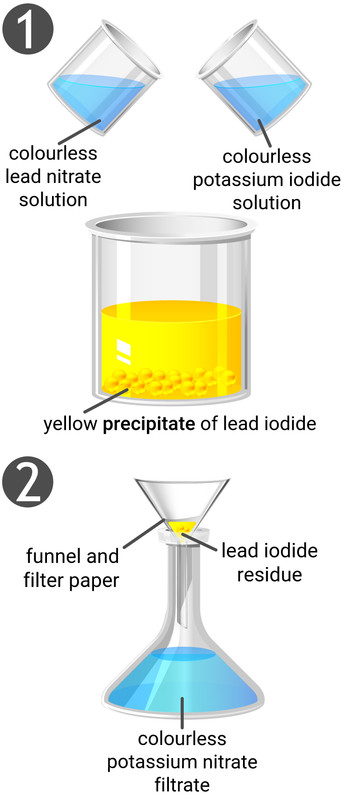
Making Insoluble Salts
An insoluble salt can be prepared by reacting two soluble salts together to form a precipitate (insoluble salt).
Method
- Mix together two suitable solutions (see solubility rules table to determine which soluble salts will produce an insoluble salt)
- Use filtration to separate the precipitate as a residue from the solution
- Wash the precipitate with distilled water whilst it is still on the filter paper
- Leave the precipitate on the filter paper in a warm oven to dry
Solubility Rules
| soluble in water | insoluble in water |
|---|---|
| all common sodium, potassium and ammonium salts |
|
| all nitrates |
|
| most common chlorides |
silver chloride, lead chloride |
| most common sulfates |
lead sulfate, barium sulfate, calcium sulfate |
| sodium carbonate, potassium carbonate, ammonium carbonate |
most common carbonates |
| sodium hydroxide, potassium hydroxide, ammonium hydroxide |
most common hydroxides |