
Dynamic Equilibrium
Separate Chemistry 1
Dynamic equilibrium
Equilibrium is reached when the forward and reverse reactions, of a reversible reaction, occur at the same rate (the reaction must be in a closed system).
When the forward and reverse rates balance, and are equal, it can appear as if the reaction isn't doing anything - and is finished. What actually is happening is that the reactants are being converted into products at the same rate the products are converted back into reactants.
The concentrations of the reactants vs products are not necessarily equal, and there is usually more of one than the other:
- if the concentration of the reactants is higher than products, we say the equilibrium lies to the left
- if the concentration of the products is higher than the reactants, we say the equilibrium lies to the right
Dynamic equilibrium is when the forward and reverse reactions are still occurring (dynamic), but the substances remain in balance (equilibrium).
Higher Tier
If a system is at equilibrium and a change is made to any of the conditions (temperature, concentration, pressure), then that system will respond to counteract the change.
This is called Le Chatelier's Principle.
Changing the concentration, temperature and pressure of a reaction system can make a big change to where the equilibrium lies, and industry uses this principle regularly to increase the amount of product they make (for the best profits!).
Changing concentration
- if you add more reactant, the equilibrium will shift to the right to reduce the concentration of reactant (and make more product)
- if you remove some of the product, the equilibrium will shift to the right to increase the concentration of the product
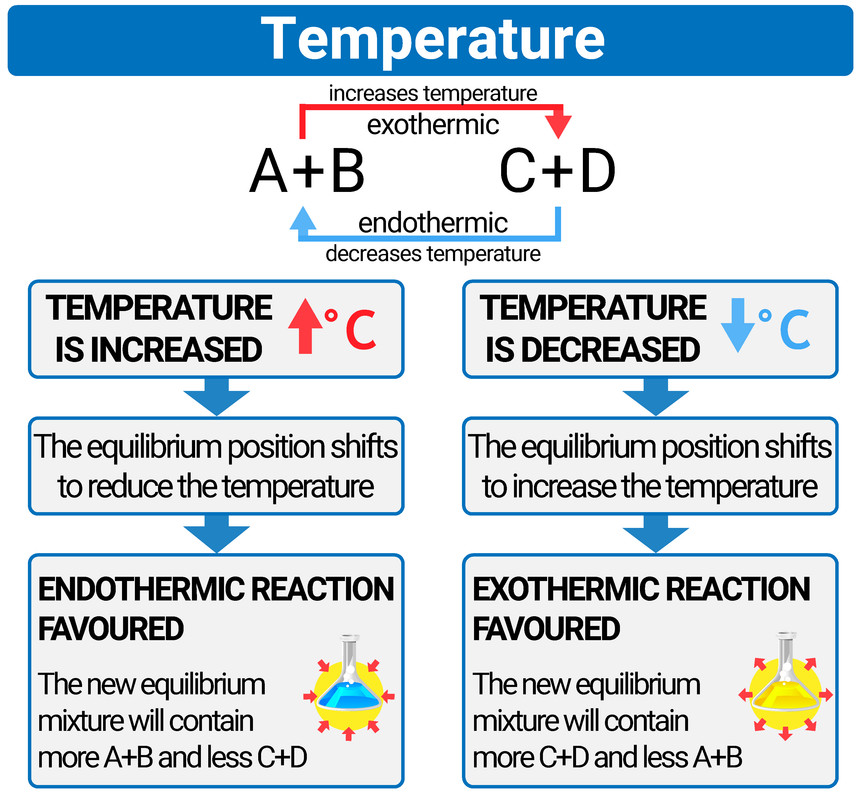
Changing temperature
- if the temperature is increased then the equilibrium position will shift to reduce the temperature (so will favour the endothermic reaction)
- if the temperature is decreased then the equilibrium position will shift to increase the temperature (so will favour the exothermic reaction)
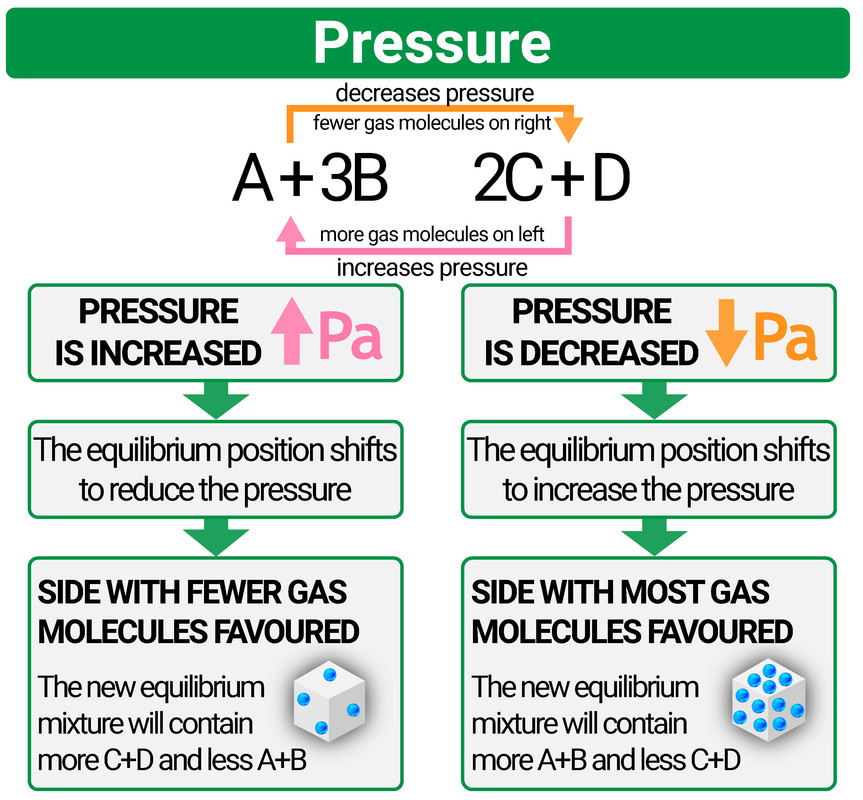
Changing pressure
- if you increase the pressure then the equilibrium will shift to reduce it (by favouring which ever side of the reaction has the fewest molecules of gas)
Adding a catalyst
- catalysts will increase the rate of both the forward and backwards reactions equally (so will not change the equilibrium position)

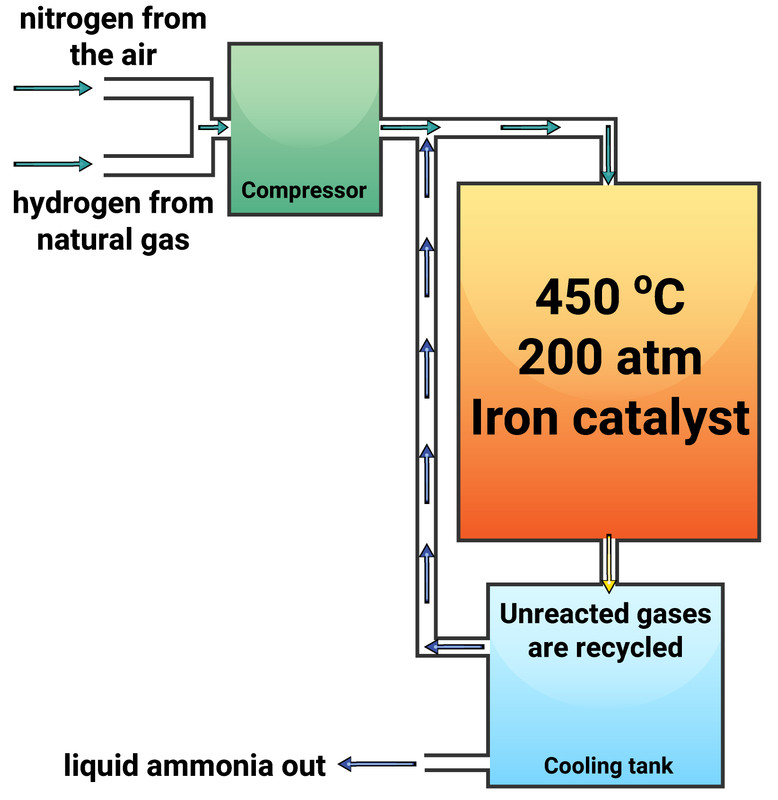
The Haber Process
nitrogen + hydrogen ⇌ ammonia
N2 + 3H2 ⇌ 2NH3
The Haber Process is used to produce ammonia, which can be used to manufacture nitrogen-based fertilisers. This process is a reversible reaction between nitrogen (extracted from the air) and hydrogen (obtained from natural gas). This reaction can reach a dynamic equilibrium.
The conditions for the Haber Process are:
- temperature of 450 °C
- pressure of 200 atmospheres (200 atm)
- iron catalyst (doesn't change the equilibrium, just increases rate of both forward and backwards reactions)
These conditions are a compromise to keep the costs down, but also to be the right conditions to achieve an acceptable yield.
Higher Tier
During industrial reactions, including the Haber process, conditions used are related to:
- the availability and cost of raw materials and energy supplies
- the control of temperature, pressure and catalyst used produce an acceptable yield in an acceptable time
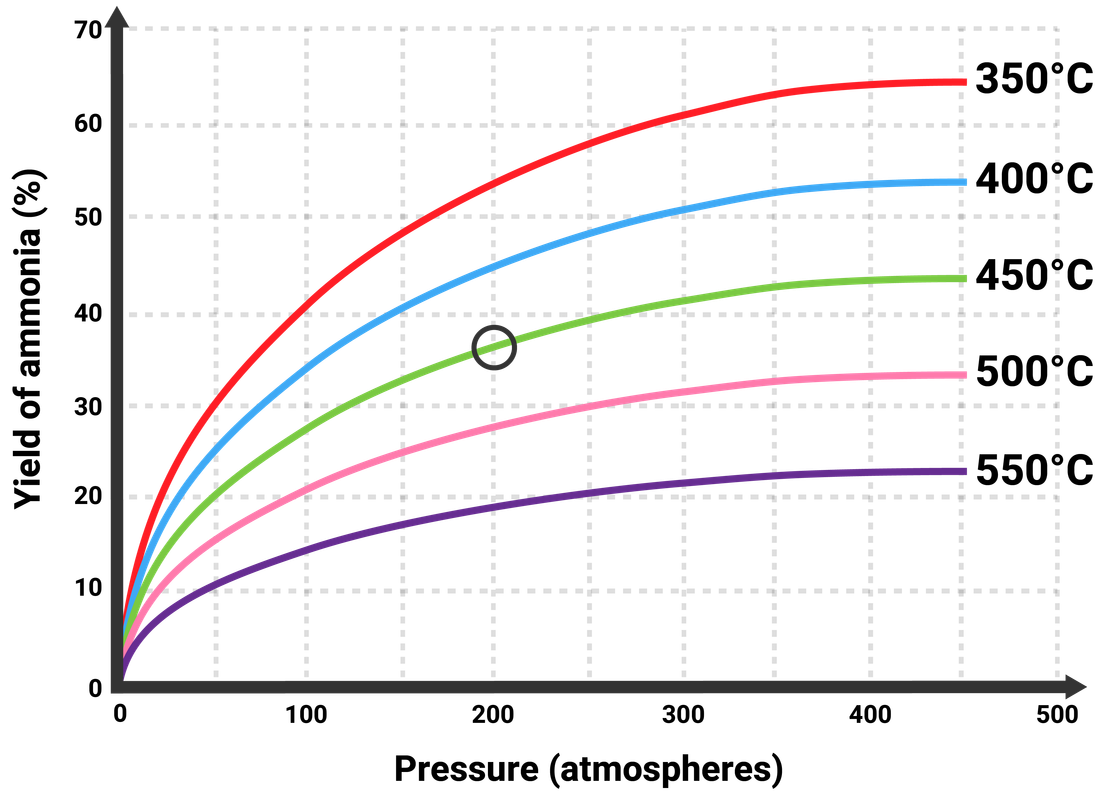
Temperature of 450 °C
- forward reaction is exothermic, so a lower temperature favours the forward reaction to maximise yield
- through heating particles will have more energy, so more frequent, successful collisions between particles (so a faster rate)
- a compromise is made on the temperature
Pressure of 200 atm
- the forward reaction turns 4 moles ('volumes') of gas into 2 moles of gas
- increasing the pressure will favour the forward reaction to maximise the yield (as this forces the particles to take up less volume)
- to maintain this high pressure, lots of energy is needed - so high costs
NPK Fertilisers
Fertilisers are made from compounds of nitrogen (for leaf development), phosphorus (aids root growth) and potassium (vital for disease resistance and root growth), and are used to improve agricultural productivity. NPK fertilisers contain compounds of all three elements. Industrial production of NPK fertilisers can be achieved using different raw materials in several different steps. NPK fertilisers are formulations of salts containing defined percentages of these elements.
Ammonium nitrate
Ammonia is made in the Haber process, and is used to manufacture ammonium salts as well as nitric acid.
Nitric acid is made from ammonia. Several stages are involved, but overall the process is:
ammonia + oxygen → nitric acid + water
NH3(g) + 2O2(g) → HNO3(aq) + H2O(l)
Ammonium nitrate is a salt used as a fertiliser. It is made by reacting ammonia with nitric acid:
ammonia + nitric acid → ammonium nitrate
NH3(g) + HNO3(aq) → NH4NO3(aq)
Making ammonium sulfate in the lab
Ammonium sulfate can be made in the lab using dilute ammonia solution and dilute
sulfuric acid. Both reactants are soluble, so a titration must be used to carry out this reaction:
ammonia + sulfuric acid → ammonium sulfate
2NH3(aq) + H2SO4(aq) → (NH4)2SO4(aq)
The lab preparation of ammonium sulfate is a batch process. This means that only a small amount of product at any one time, and the apparatus needs to be cleaned before each new batch.
Method
- pour dilute sulfuric acid into a beaker
- add a few drops of methyl orange indicator
- add dilute ammonia solution drop by drop, stirring in between each addition
- continue step 3 until the colour permanently changes from red to orange
- pour the reaction mixture into an evaporating basin, and heat carefully over a boiling water bath
- stop heating before all the water has evaporated. Leave aside for crystals to form
- pour away excess water and leave the crystals to dry in a warm oven (or pat dry with filter paper)
| Laboratory preparation |
Industrial preparation |
|
|---|---|---|
| scale | small | large |
| starting materials |
ammonia solution and dilute sulfuric acid | raw materials for making ammonia and sulfuric acid |
| stages | titration, then crystallisation | many |
| process | batch | continuous |
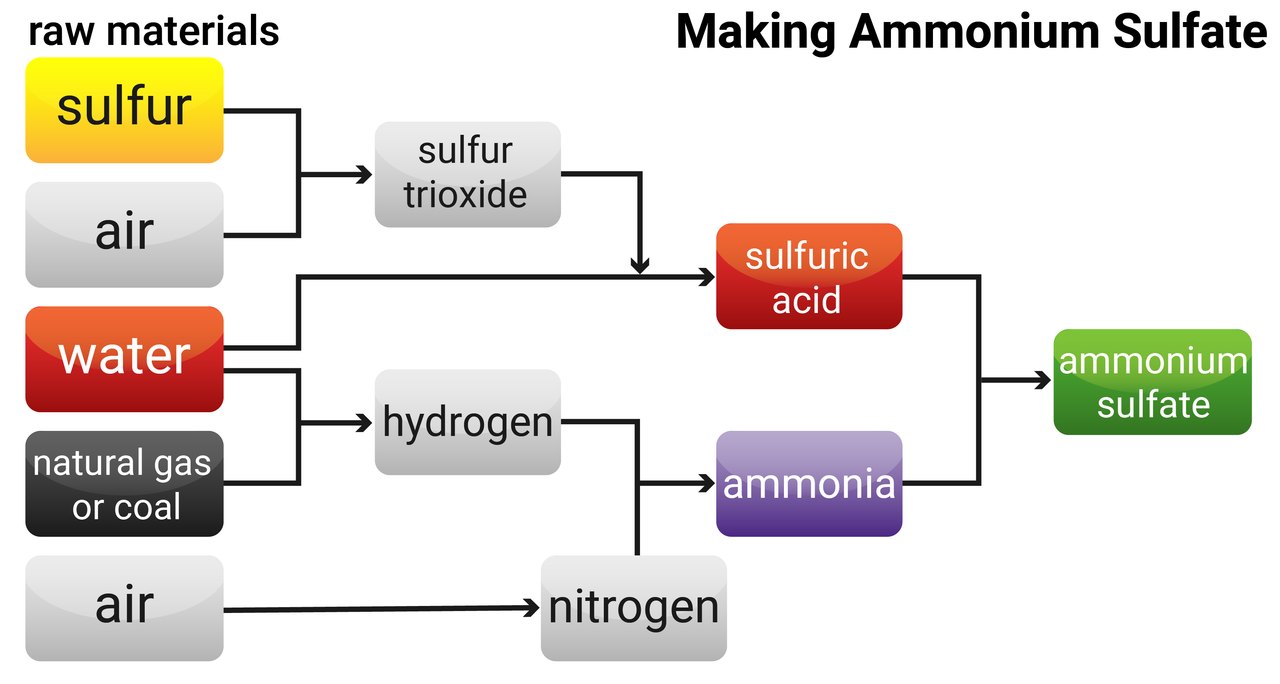
Industrial production of ammonium sulfate
The industrial production of ammonium sulfate happens on a much larger scale than its production in the lab. A fertiliser factory begins with the raw materials needed to make ammonia and sulfuric acid, rather than buying these two reactants from elsewhere.
The industrial production of ammonium sulfate is a continuous process. The product is made quickly all the time, as long as raw materials are provided.