
Transition Metals, Alloys and Corrosion
Separate Chemistry 1
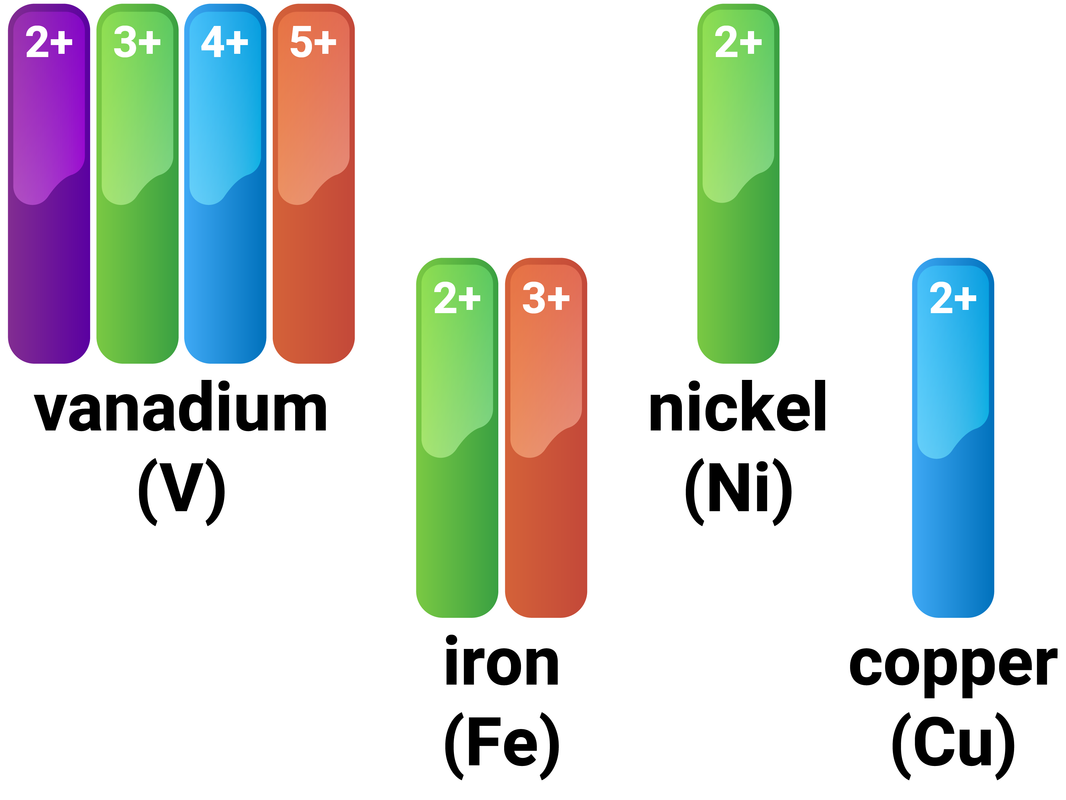
Transition Metals
Most metals on the Periodic Table are classed as transition metals, and these metals typically:
- are malleable (can be hammered or rolled into shape without shattering)
- are ductile (can be stretched out to make thin wires)
- have high melting points
- have high density
- are good conductors of electricity and heat
- are shiny when polished
- form coloured compounds
- have catalytic activity as a pure metal, and as a compound.
Compared to aluminium and the group 1 and 2 metals, transition metals typically have higher melting points, and higher densities.
Iron (Fe) as an example
Iron follows these general rules for transition metals, and also has a very high melting point (1538°C) and a high density (7.87 g/cm³). It can form many coloured compound, including:
- iron(II) hydroxide - pale green
- iron(III) hydroxide - orange/brown
- iron(III) oxide - red/brown
Iron is also used as a catalyst in the Haber Process to make ammonia, and iron(III) oxide is used to make hydrogen by reacting carbon monoxide with steam.
Corrosion
Corrosion is the destruction of materials by chemical reactions with oxygen in the air. When metals corrode they are oxidised, to form metal oxides. Metals may form a thin layer of tarnish when they oxidise. This layer stops oxygen reaching the metal, preventing further oxidation.
Rusting is a specific example of corrosion, and requires both air and water for iron (or steel) to rust. There are three ways to prevent iron from rusting:
- exclusion of oxygen (by either using an atmosphere of nitrogen or argon)
- exclusion of water (by storing with a desiccant)
- sacrificial protection (when a more reactive metal than iron is attached to the object to prevent iron from rusting)
-
galvanising is a specific example of scarifical protection where a thin layer of zinc coats iron or steel, and can be carried out either be electrolysis or dipping the object in molten zinc
Corrosion can also be prevented by using a physical barrier (e.g. applying a coating such as greasing, painting, or plastic).
Electroplating
Electrolysis can be used to put a thin layer of a metal on an object that you don't want to corrode. An example is steel cutlery being electroplated with silver. It requires:
- the cathode to be the object you want electroplated (e.g. steel cutlery)
- the anode to be the metal you are plating the object with (e.g. silver)
- the electrolyte to contain ions of the metal found at the anode (e.g. silver nitrate solution)
Electroplating is used to improve the corrosion resistance of metal objects, but also is used to improve appearance (e.g. gold-plating jewellery).
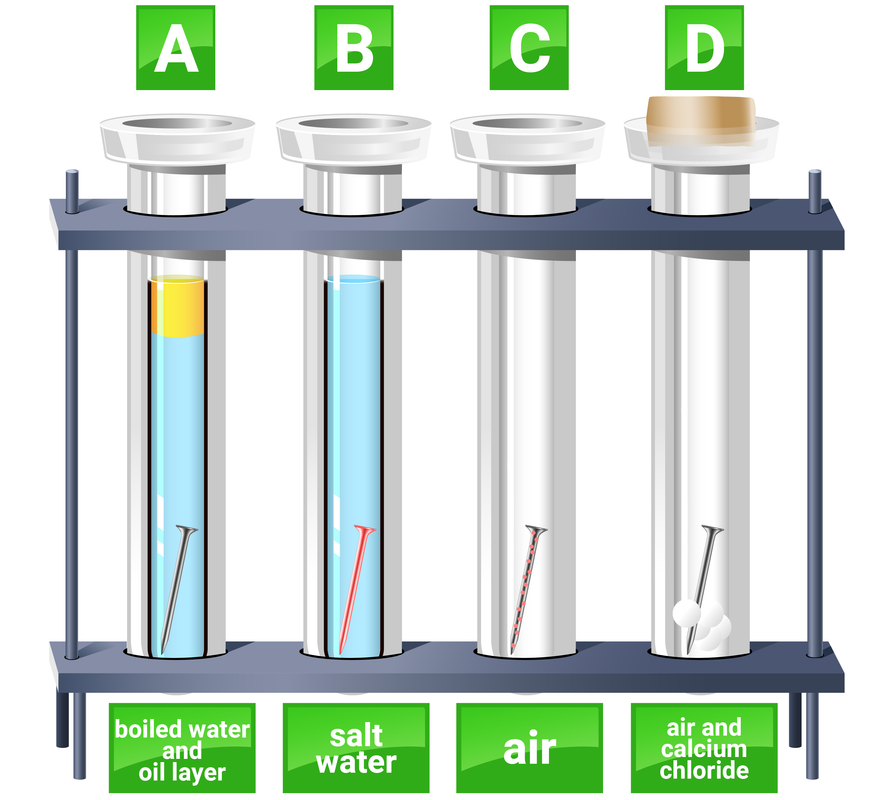 Different conditions demonstrating if an iron nail would corrode.
Different conditions demonstrating if an iron nail would corrode.
Alloys
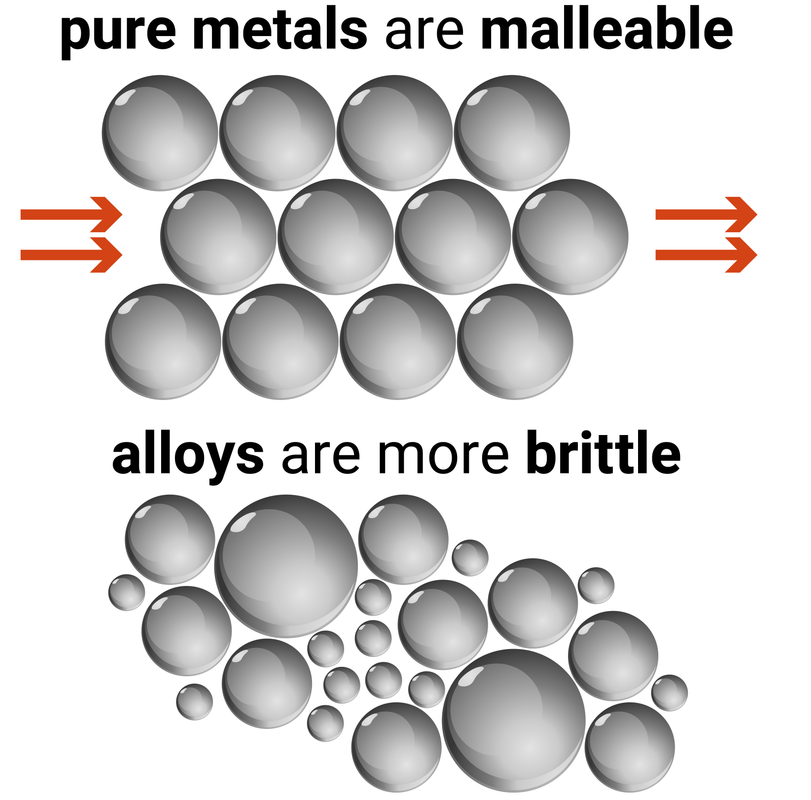
Most metals in everyday use are alloys. An alloy is a mixture of two or more elements, where at least one of the elements is a metal.
Pure metals are generally too soft to use as they have a regular lattice structure. When a force is applied to the metal, the regularly aligned layers are able to easily slide over each other (this makes them malleable).
Alloys are made of different sized atoms, distorting the regular layers so that they are not able to easily slide over each other any more - increasing the strength of the metal (they are less malleable, and more brittle).
Alloy steels
Iron is able to be alloyed with other elements to produce a variety of alloy steels. These alloys have different properties depending on which elements, and how much of each element, is present.
| steel | element | properties | use |
|---|---|---|---|
| mild | carbon | malleable, ductile | car body parts |
| tool | tungsten | hard, resistant to high temperatures | drill bits |
| stainless | chromium | hard, resistant to rusting | taps, cutlery |
Uses of metals and alloys
Aluminium, gold and copper are useful metals, and the uses of these metals is related to their properties.
Aluminium does not react with water, as its surface is protected by a naturally formed layer of aluinium oxide (allows the metal to resist corrosion). Aluminium foil is used domestically to wrap/store food, because:
- it does not react with the substances in food
- it is malleable and so can easily be folded around the food
- it has a low density so it is very lightweight
Aluminium, gold and copper can also be useful as alloys, some of which are shown below:
| alloy | composition |
|---|---|
| magnalium | aluminium and magnesium |
| brass | copper and zinc |
| jewellery gold | gold and copper |
Magnalium is stronger than pure aluminium, but it has a low density so it used to make aircraft parts.