Atoms, Elements and Compounds
Atomic Structure and the Periodic Table
Atoms, Elements and Compounds
All substances are made of atoms. An atom is the smallest part of an element that can exist.
Atoms of each element are represented by a chemical symbol, for example:
- O represents an atom of oxygen
- Na represents an atom of sodium
There are about 100 different elements. Elements are shown in the Periodic Table.
Compounds are formed from elements by chemical reactions. Chemical reactions always involve the formation of one or more new substances, and often involve a detectable energy change. Compounds contain two or more elements chemically combined in fixed proportions and can be represented by formulae using the symbols of the atoms from which they were formed. Compounds can only be separated into elements by chemical reactions.
Chemical reactions can be represented by word equations or equations using symbols and formulae.
Separation Techniques
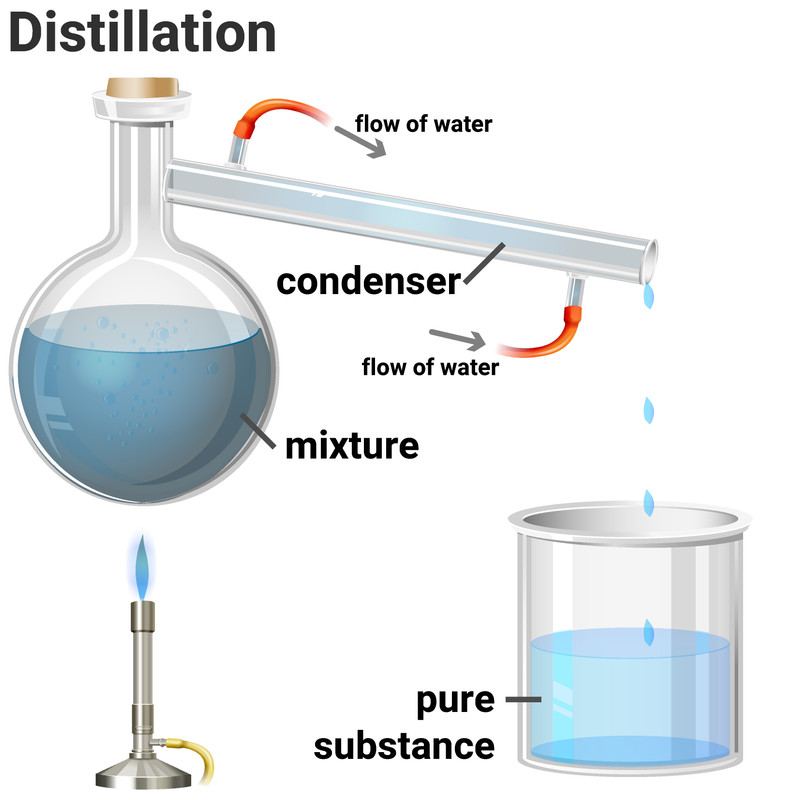
Simple distillation is a method for separating the solvent from a solution, leaving behind the solute. This method works because the solvent has a much lower boiling point than the dissolved solute. Heating the solution allows the solvent to evaporate, and then it passes through a condenser, where it is cooled and condensed into a separate container. The solute does not evaporate and so it stays behind.
Fractional distillation is a method of separating multiple liquids from each other. The technique works in the same way as distillation, but on a much larger scale.
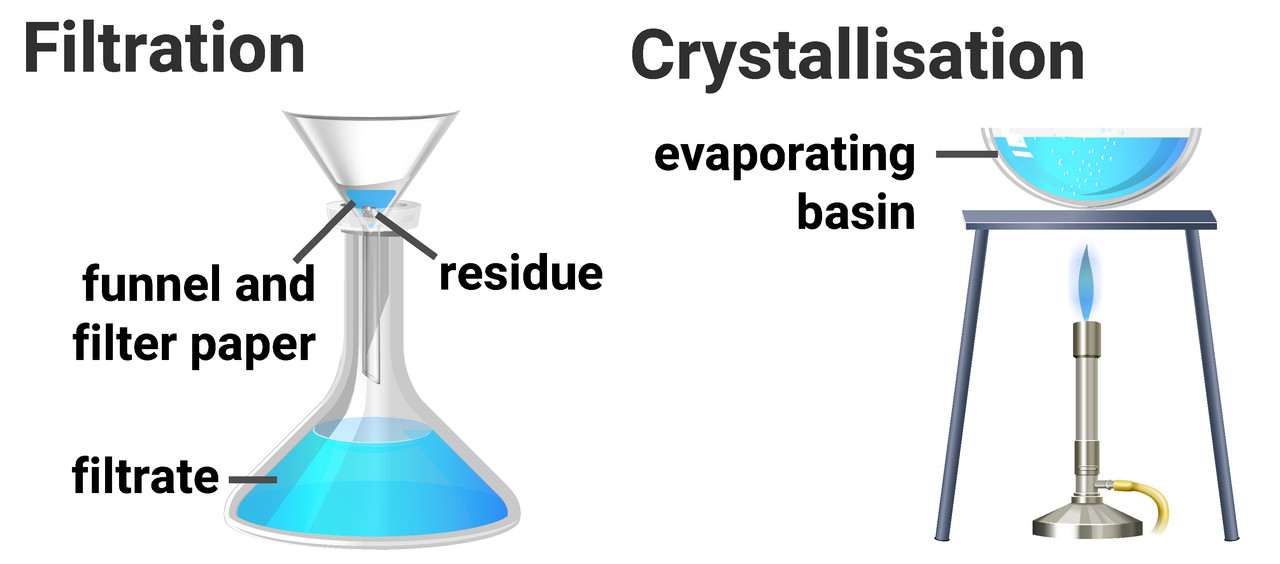
Filtration is a method for separating an insoluble solid from a liquid. A mixture of liquid and solid is passed through filter paper into a flask below. The solid stays on the filter paper (residue), and the liquid passes through to the container below (filtrate).
Crystallisation/evaporation is a method that separates a soluble solid from a liquid. A solution of liquid and dissolved solid is heated, causing the solvent to evaporate and leaves solid crystals behind.
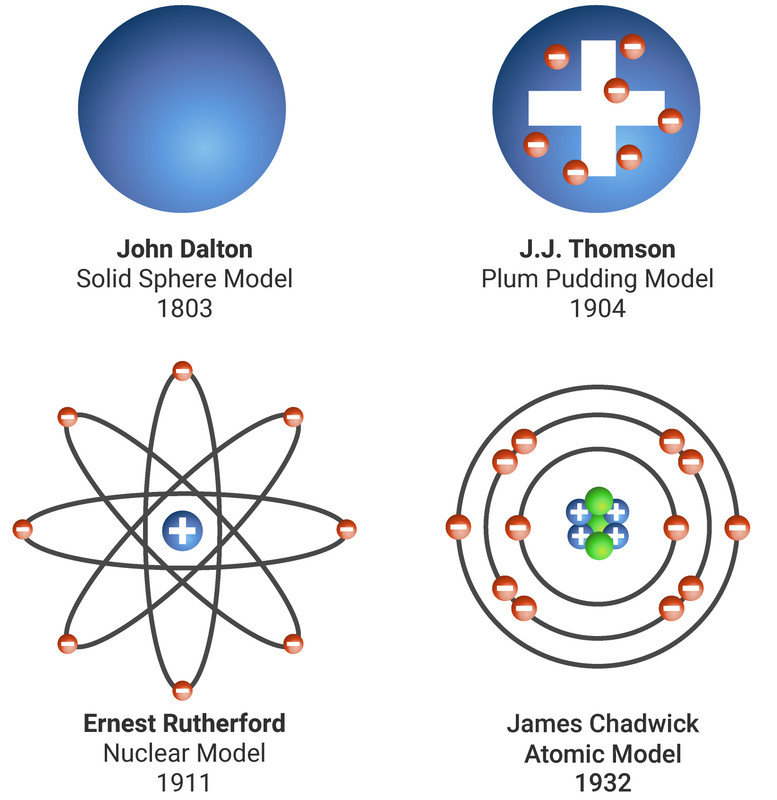
Atomic History
New experimental evidence may lead to a scientific model being changed or replaced.
John Dalton thought that all matter was made of tiny particles called atoms, which he imagined as tiny spheres that could not be divided.
J.J. Thomson discovered the electron. He suggested a plum pudding model of the atom. In this model the atom is a ball of positive charge with negative electrons randomly distributed within it.
Ernest Rutherford designed an experiment to test the plum pudding model. In the experiment he fired positively charged alpha particles at thin gold foil. Most alpha particles went straight through the foil, but a few were scattered in different directions or were reflected back. From his results he made two conclusions:
- the mass of an atom is concentrated at its centre, the nucleus (which is very small)
- the nucleus is positively charged
Niels Bohr adapted Ernest Rutherford's model. Bohr did calculations that led him to suggest that electrons orbit the nucleus in fixed energy levels - called shells. The shells are at certain distances from the nucleus.
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge. The name proton was given to these particles.
James Chadwick found evidence for the existence of particles in the nucleus with mass but no charge. These particles are called neutrons.
Atomic Structure
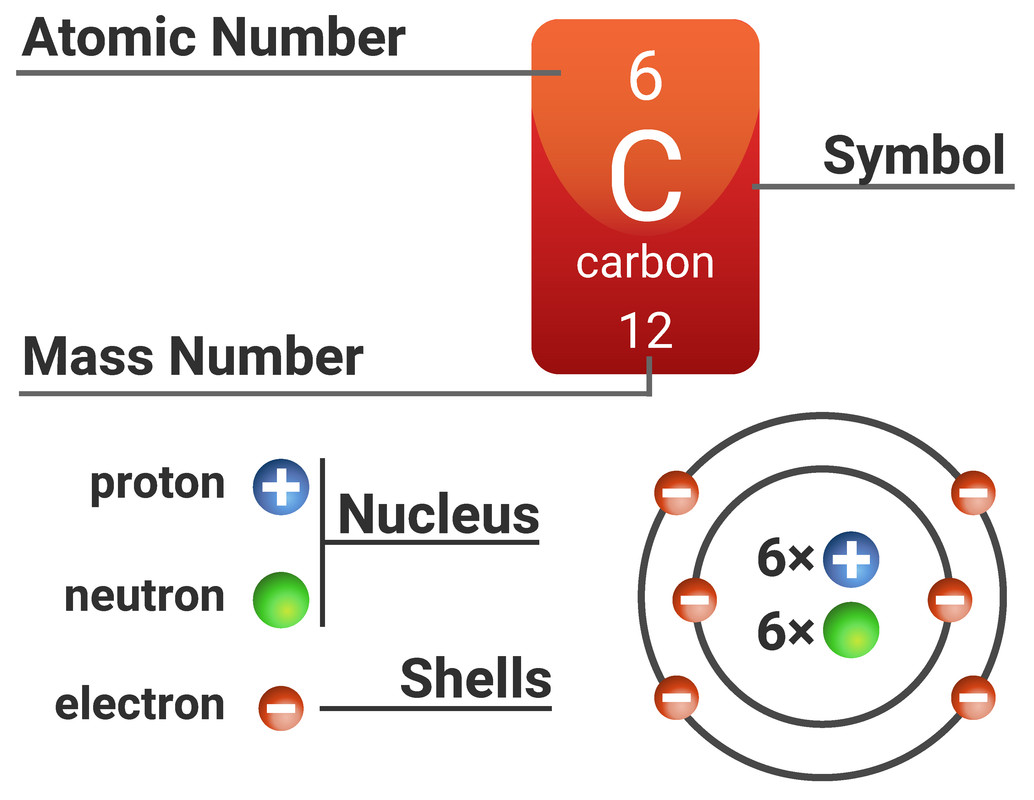
Atoms are made from three types of subatomic particles: protons, neutrons and electrons.
On the Periodic Table you will find every element that we know about, which in turn is a list of every atom that we have discovered.
Next to each element's symbol you will find several numbers:
- The atomic number - this is the number of protons
- The mass number - this is the total number of protons and neutrons
| name of particle |
mass (relative) |
charge (relative) |
location in atom |
|---|---|---|---|
| proton | 1 | +1 | nucleus |
| neutron | 1 | 0 | nucleus |
| electron | very small | -1 | shells |
Every atom of the same element has the same number of protons as electrons. This is because every atom is neutral.
An atom has a radius of about 0.1 nm, with about 99% of an atom being empty space! The radius of a nucleus is less than 1/10000 (that's really small!) of that of the atom. Almost all of the mass of an atom is in the nucleus.
The proton number of an element defines the element. Every atom of one element must have the same number of protons.
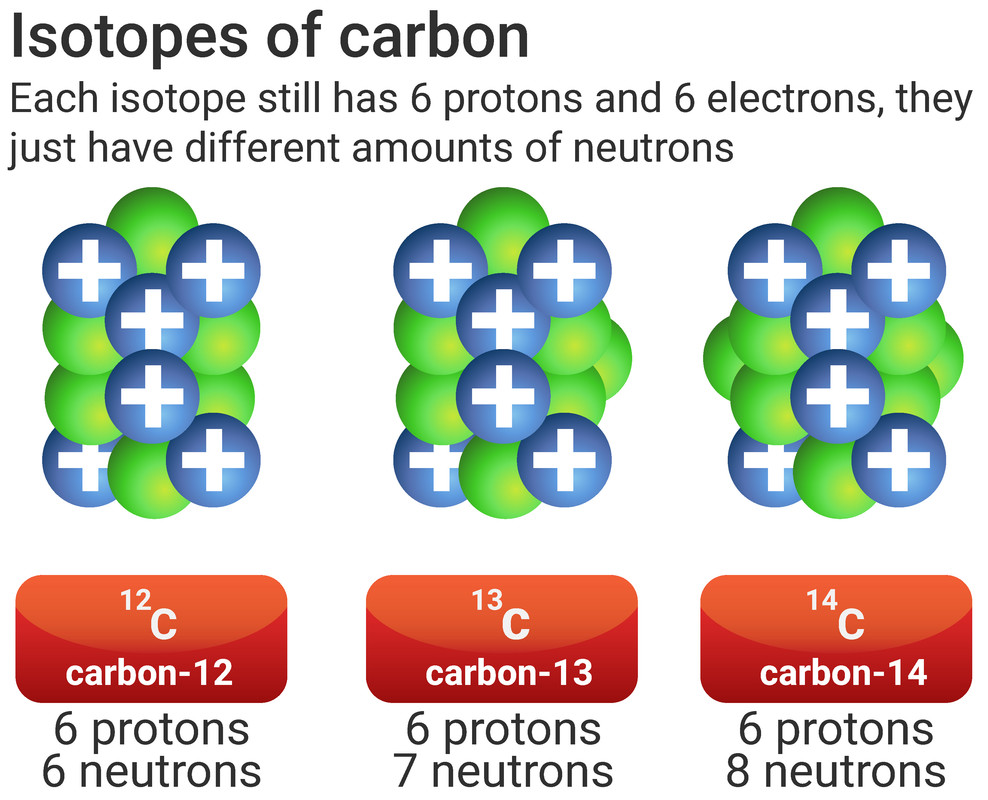
Isotopes
All atoms will always have the same number of protons as they do electrons. This is because atoms are neutral, and this is achieved by having the same number of positive charges (protons) and negative charges (electrons).
Not all atoms will have the same number of neutrons. All atoms of carbon have 6 protons, making its atomic number 6. Most of those carbon atoms will also have 6 neutrons, making the mass number 12 (6+6). However, some of those atoms of carbon will have a different number of neutrons, making the mass number different too.
Isotopes are different forms of the same element, they have:
- the same atomic number (same number of protons and electrons)
- a different mass number (different number of neutrons)
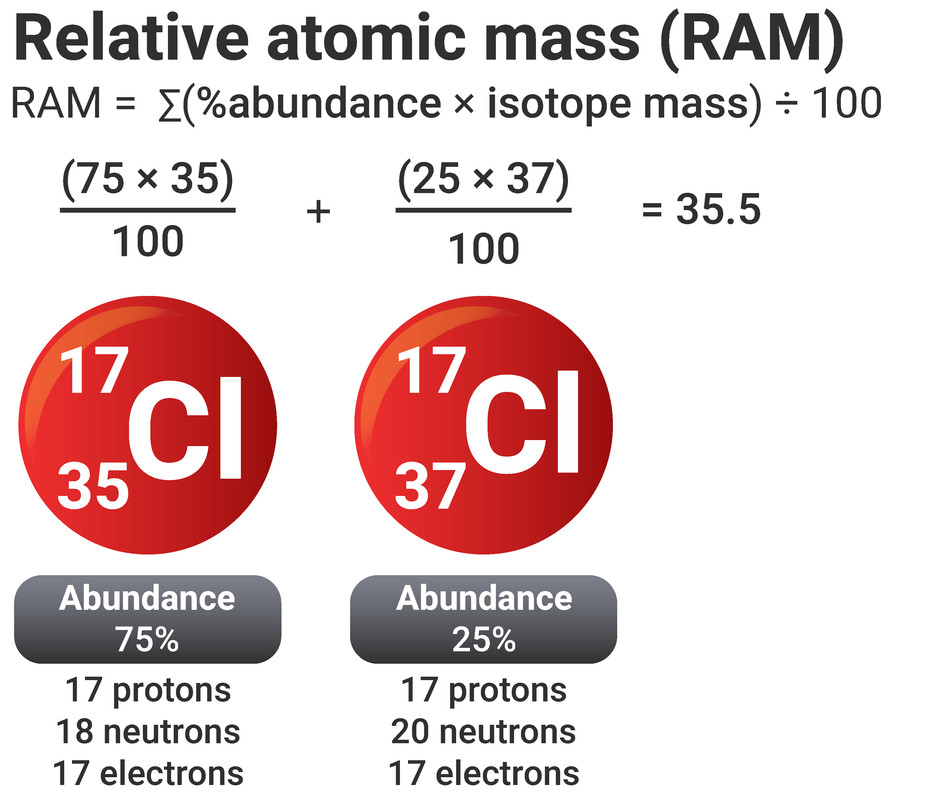
Not all atoms have a whole number mass. Chlorine, as an example, has a recorded mass of 35.5. This is because we have to take the masses of all an element's isotopes into account.
We can use the percentage abundances (how common it is) and the isotopes' masses in the equation below to calculate a relative atomic mass.
relative atomic mass = ∑(%abundance × isotope mass) ÷ 100
Electron Configurations
Electrons orbit around an atom in a certain pattern. Around the nucleus are different energy levels, often called shells. Each shell can be filled to a specific number of electrons, and these limits are the same for every element. Each shell must be filled before another shell can take electrons.
Atoms will only fill to a maximum of:
- 2 electrons in the first shell,
- 8 in the second shell,
- 8 in the third shell
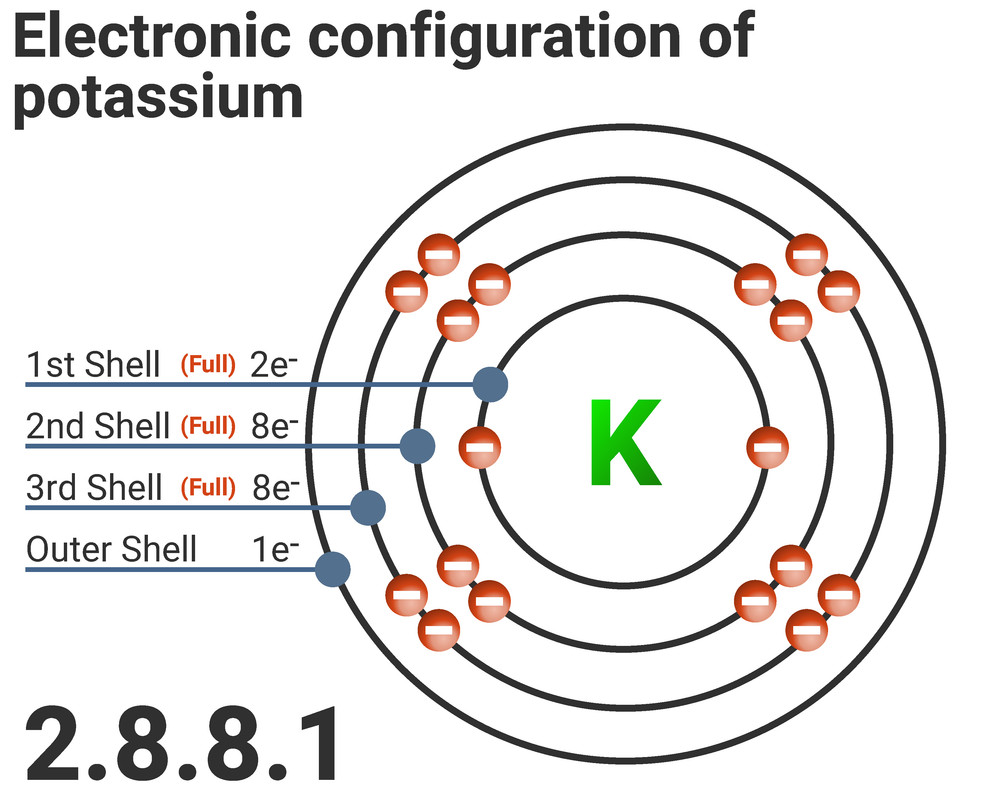
We can either write, or draw, the electron configuration of an atom. Here you can see the electron configuration of potassium.
Potassium:
- is in period 3 - so has 3 shells of electrons
- is in group 1 - so has 1 electron on its outermost shell
- has a configuration of 2.8.8.1 because all shells must be filled before a new shell can contain electrons