
The Periodic Table
Atomic Structure and the Periodic Table
History of the Periodic Table
Before the discovery of protons, neutrons and electrons, scientists attempted to classify the elements by arranging them in order of their atomic weights.
The early periodic tables were incomplete and some elements were placed in inappropriate groups if the strict order of atomic weights was followed.
Mendeleev overcame some of the problems by leaving gaps for elements that he thought had not been discovered and in some places changed the order based on atomic weights. Mendeleev is most famous for leaving these gaps, claiming they were due to undiscovered elements. For some of these elements, he predicted their properties and masses... and got them right!
Elements with properties predicted by Mendeleev were later discovered and filled the gaps. Our knowledge of isotopes made it possible to explain why the order based on atomic weights was not always correct.
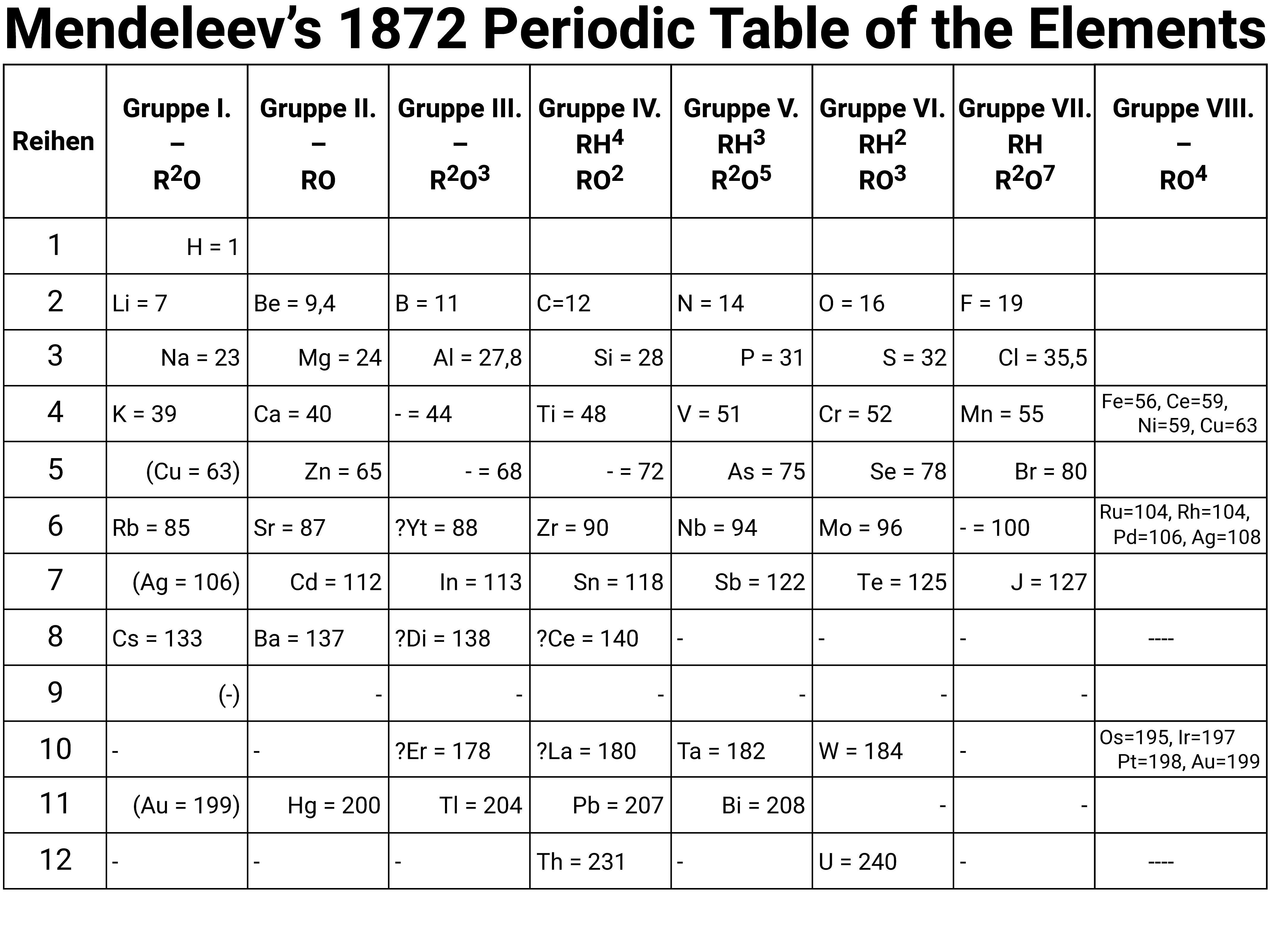
Mendeleev's Table (Key)
The symbols R²O and RH⁴, use superscripts to show the number of atoms in molecules rather than the current style of using subscripts. For group 1 ("Gruppe I") this would mean they could form molecules like H²O, and Li²O ... which we know today is correct.
The gaps marked with hyphens show elements deduced by Mendeleev as existing, but unknown in 1872; he predicted the properties of some of these elements.
John Newlands
Four years before Mendeleev published his version of the table, Newlands noticed patterns of similar properties between elements with atomic weights every eight elements. He called this The Law of Octaves.
The Periodic Table
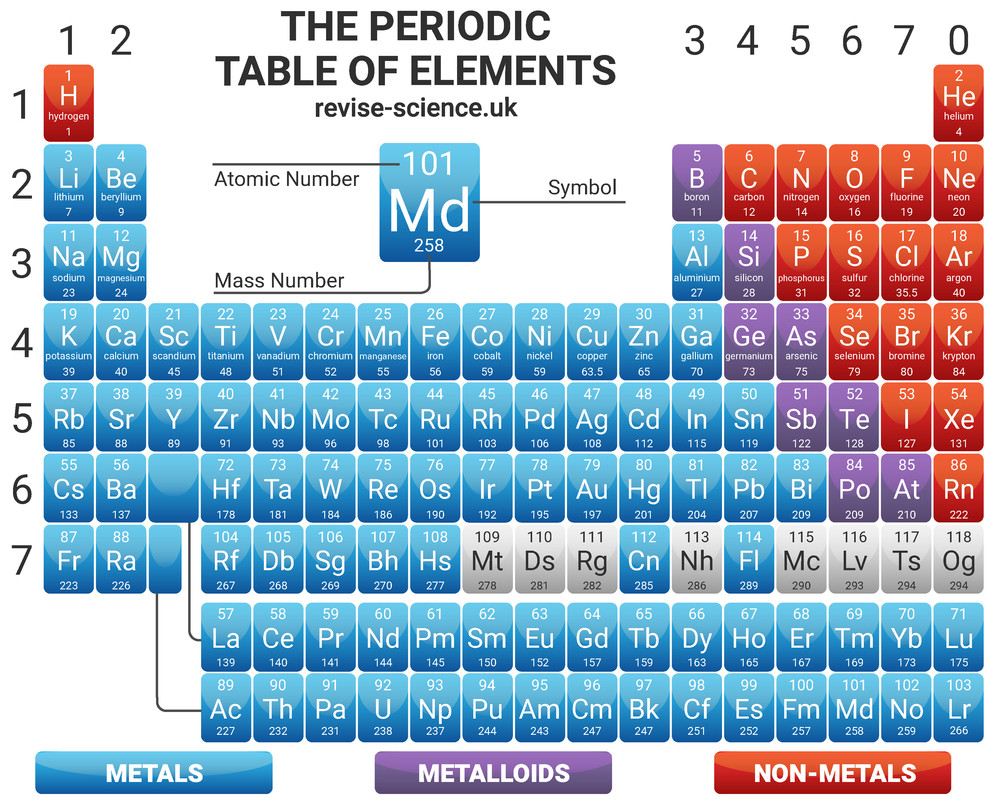
On the Periodic Table you will find every element that we know about, which in turn is a list of every atom that we have discovered. It is ordered by atomic number, meaning that reading from left to right increases the proton number by one every time.
The Periodic Table is arranged in such a way that elements with similar properties can be found together in columns called 'groups' - for example the group 1 metals are all very reactive with water. Elements in the same group have the same number of electrons in their outershell (these are known as valence electrons).
Elements in the same period have the same number of electron shells.
The Periodic Table is split with metals all found on the left side of the table, and non metals found on the right. Metals have either 1, 2 or 3 electrons on their outermost shell, so want to lose electrons to form positive ions (cations). Non-metals that have either 5, 6 or 7 electrons on their outermost shell want to gain electrons to form negative ions (anions). Non-metals with 8 electrons, the Noble Gases, don't lose or gain electrons to form ions, as they are stable (and therefore inert).
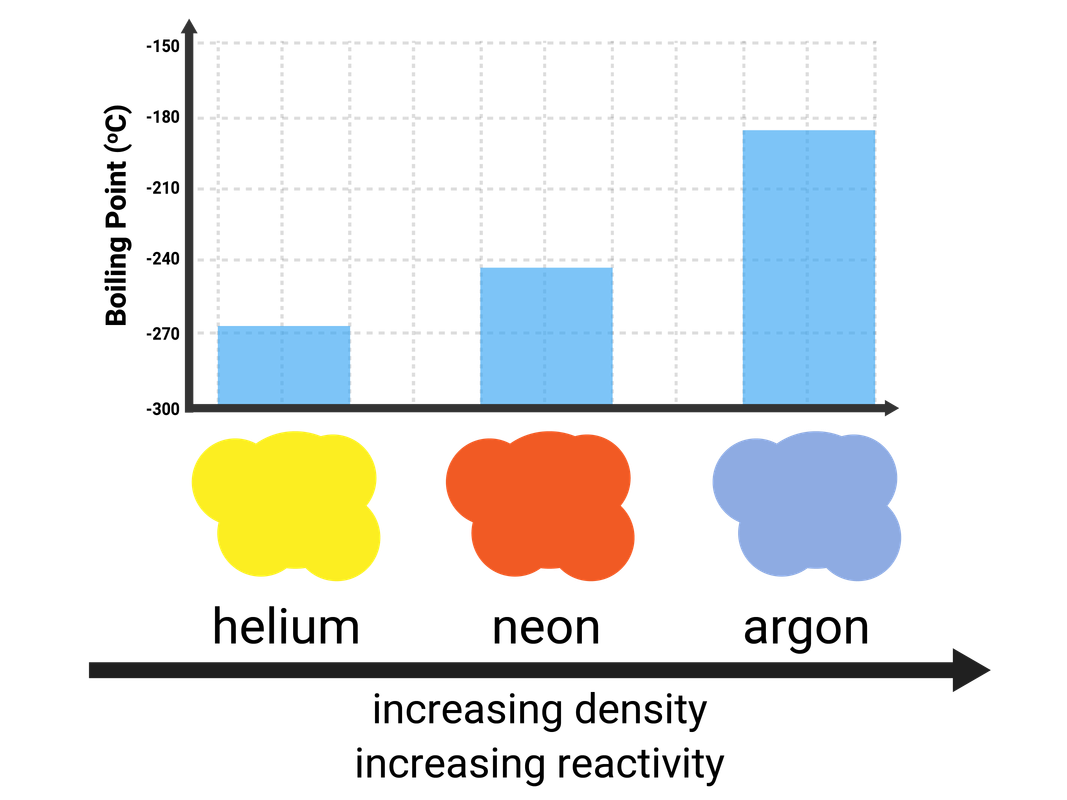
Group 0 (Noble Gases)
Helium, neon, argon, krypton, and xenon are all Noble Gases, because they have full outershells of electrons and are stable. This means they are unreactive, as they do not need to react to gain electrons - so they are called inert.
The noble gases have eight electrons in their outer shell, except for helium, which has
only two electrons.
These gases are all colourless.
All of the elements in this group are gases at room temperature, as they have very low melting and boiling points. The boiling points do increase down the group, and this is due to stronger intermolecular forces of attraction (meaning more energy is needed to break them).
The noble gases have many uses, mainly due to them being so unreactive (and therefore non-flammable) and their low density. Some examples:
- helium is used in balloons (due to low density and being non-flammable)
- argon is used as a 'shield gas' when welding pieces of metal together (denser than air, and stops the metal oxidising)
- when electricity is passed through these gases they glow different colours:
- helium glows yellow
- neon glows orange-red
- argon glows blue-lilac
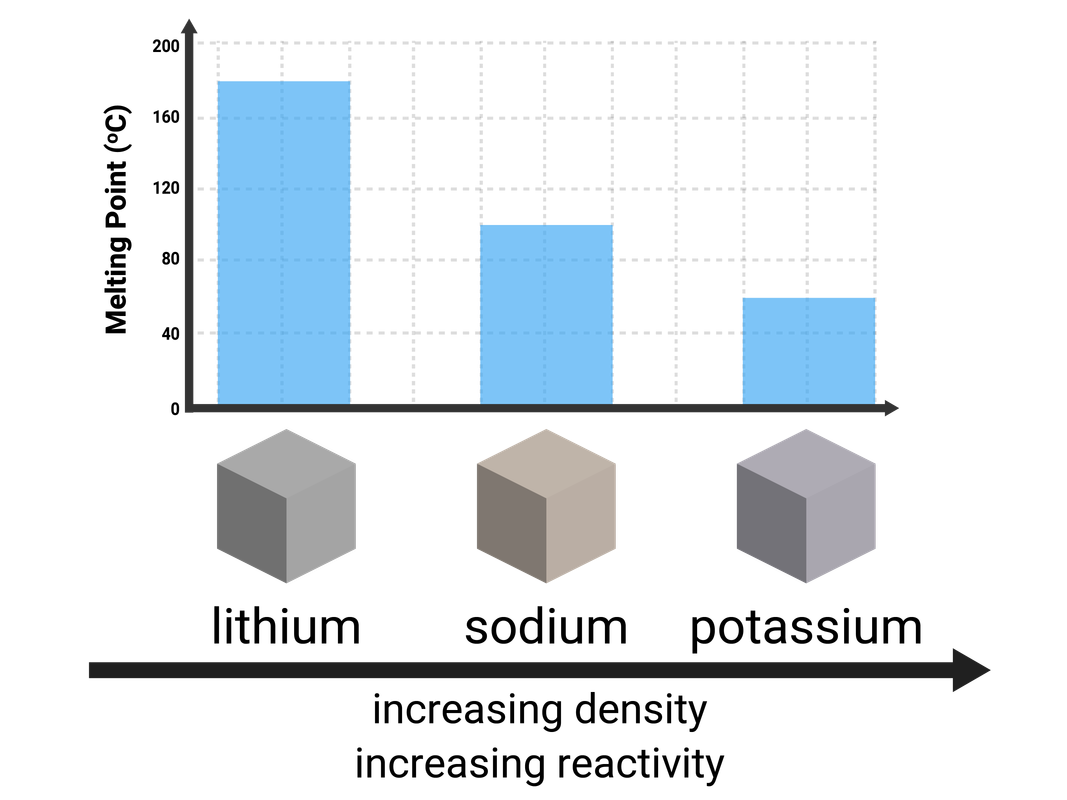
Group 1 (Alkali Metals)
Lithium, sodium, potassium, rubidium, and caesium are all found in Group 1, and are commonly known as the Alkali Metals. Every atom of these elements contains 1 electron on their outermost shell.
These metals are unusual as they:
- are very soft (can be cut with a knife)
- have relatively low melting points, that decrease down the group
These metals have to be stored in oil, as they oxidise in air very easily. We can see this by cutting into the metal: it is shiny on the inside, but dull on the outside.
Group 1 metals will react with water to form an alkaline solution:
- lithium + water → lithium hydroxide + hydrogen
- sodium + water → sodium hydroxide + hydrogen
- potassium + water → potassium hydroxide + hydrogen
Alkali metals become more reactive, the larger the atom. This means the metals near the top of the group (lithium, sodium etc) are much less reactive than those near the bottom of the group (rubidium, caesium etc). This is because the outer negative electron is further away from the positive nucleus, meaning it doesn't feel as much attractive force and so can be more easily lost.
Group 7 (Halogens)
Fluorine, chlorine, bromine, and iodine are found in Group 7, and are commonly known as the Halogens. Every atom of these elements contains 7 electrons on their outermost shell.
At room temperature these elements in various states, and have very different colours:
| chlorine, Cl2 | bromine, Br2 |
iodine, I2 |
|---|---|---|
| pale green gas | brown liquid | purple-black solid |
Notice that moving from the top of the group (fluorine - gas) to the bottom of the group (astatine - solid), shows the melting/boiling points of these elements increases as the states they are found in at room temperature change quite significantly.
This is because:
- the molecules become larger
- the intermolecular forces between molecules become stronger
- and so more energy is needed to overcome these forces
Reactions of the Halogens
Group 7 elements will react with metals to form metal halides:
- chlorine + iron → iron fluoride
- bromine + sodium → sodium bromide
- iodine + magnesium → magnesium iodide
Group 7 elements will react with hydrogen to form hydrogen halides (which dissolve in water to form acidic solutions):
- chlorine + hydrogen → hydrogen chloride
- bromine + hydrogen → hydrogen bromide
- iodine + hydrogen → hydrogen iodide
The Group 7 elements become less reactive, the larger the atom. This means the elements near the top of the group (fluorine, chlorine etc) are much more reactive than those near the bottom of the group (iodine, astatine etc). This is because the outer negative electron shell is further away from the positive nucleus, meaning it doesn't feel as much attractive force and so is more difficult to attract an electron.
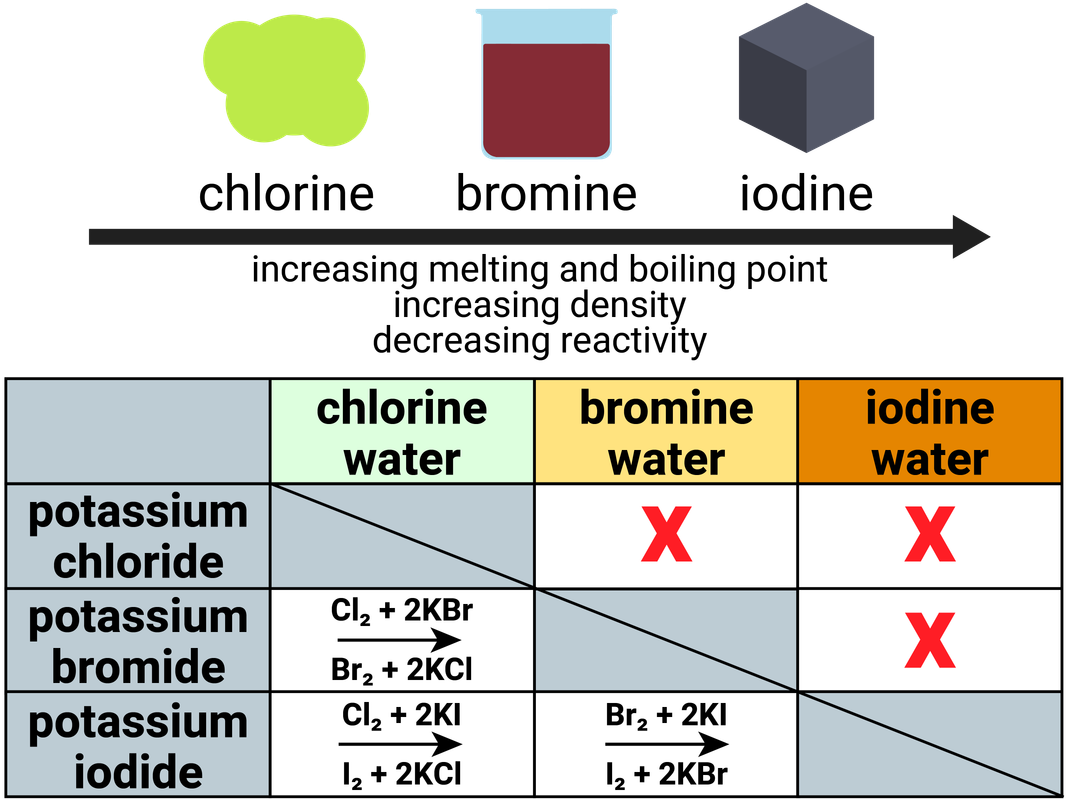
Displacement reactions
A more reactive halogen will displace a less reactive halogen in solution:
- chlorine + sodium bromide → bromine + sodium chloride
- chlorine + sodium iodide → iodine + sodium chloride
- bromine + sodium iodide → iodine + sodium bromide
Higher Tier
Displacement reactions are a type of redox chemistry, i.e. there are oxidation and reduction reactions happening. We can see this when we split up a halogen displacement equation into half equations:
chlorine + sodium bromide → sodium chloride + bromine
Cl2 + 2NaBr → 2NaCl + Br2
2Br- → Br2 + 2e-
Cl2 + 2e- → 2Cl-
In this example, bromine is oxidised (loss of electrons) and the chlorine is reduced (gain of electrons).