
Properties of Transition Metals (chemistry only)
Atomic Structure and the Periodic Table
Transition Metals
Most metals on the Periodic Table are classed as transition metals, and these metals typically:
- are malleable (can be hammered or rolled into shape without shattering)
- are ductile (can be stretched out to make thin wires)
- have high melting points
- have high density
- are good conductors of electricity and heat
- are shiny when polished
- form coloured compounds
- have catalytic activity as a pure metal, and as a compound.
Compared to aluminium and the group 1 and 2 metals, transition metals typically have higher melting points, and higher densities.
Iron (Fe) as an example
Iron follows these general rules for transition metals, and also has a very high melting point (1538°C) and a high density (7.87 g/cm³). It can form many coloured compound, including:
- iron(II) hydroxide - pale green
- iron(III) hydroxide - orange/brown
- iron(III) oxide - red/brown
Iron is also used as a catalyst in the Haber Process to make ammonia, and iron(III) oxide is used to make hydrogen by reacting carbon monoxide with steam.
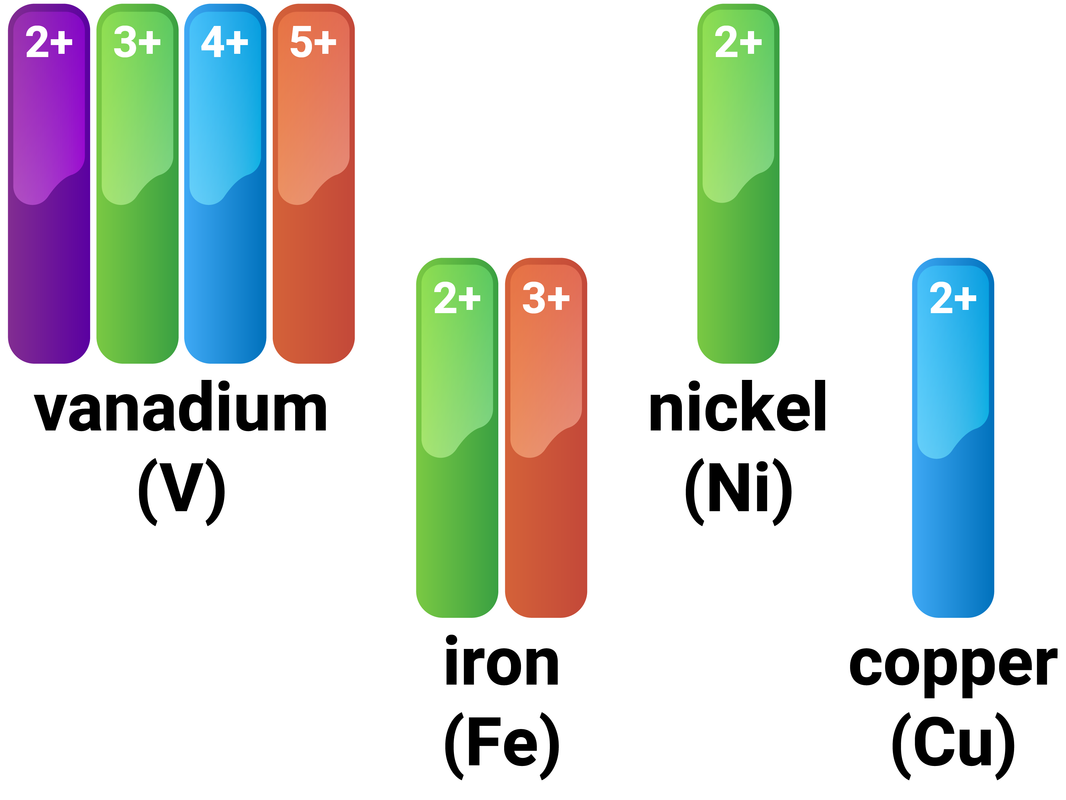
The following elements have properties that are typical of transition elements:
- chromium, Cr
- manganese, Mn
- iron, Fe
- cobalt, Co
- nickel, Ni
- copper, Cu
Reactions of Transition Metals
Not only are there differences in the physical properties of transition metals compared to group 1 metals, there are differences in the chemical properties too.
The group 1 elements react quickly with oxygen in the air at room temperature. Most transition elements react slowly, or not at all, with oxygen at room temperature.
copper + oxygen → copper oxide
Cu(s) + O2(g) → CuO(s)
The group 1 elements react vigorously with cold water. Most transition elements react slowly with cold water, or not at all.
The group 1 elements react vigorously with the halogens. Some transition elements also react with halogens.
iron + chlorine → iron(III) chloride
Fe(s) + Cl2(g) → FeCl3(s)