Carbon Compounds as Fuels and Feedstock
Organic Chemistry
Crude Oil
Crude oil is a fossil fuel, and is made up of a complex mixture of hydrocarbons. This mixture contains many useful compounds (in which carbon atoms are in chains or rings) that need to be separated using a process called fractional distillation. Crude oil can be described as a finite resource.
A hydrocarbon is a chemical that contains hydrogen and carbon atoms only. Crude oil mostly contains hydrocarbons called alkanes.
Crude oil is an important source of useful substances. The chemicals it contains can be used as either fuels, or feedstock for the petrochemical industry.
The hydrocarbon molecules in crude oil each have a different number of carbon atoms, and therefore have different chain lengths.
Properties of hydrocarbons
As the chain length of a hydrocarbon increases, the properties of the hydrocarbon change with it.
A longer hydrocarbon chain will:
- have a higher boiling point
- be less flammable (harder to ignite)
- be more viscous
The reason the boiling point and viscosity increases is due to there are more intermolecular forces of attraction holding the chains together (more energy needed to break these apart).
Fractional Distillation
Fractional distillation is the process of taking a complex mixture of liquids (like crude oil) and separating it out into simpler, more useful mixtures.
To separate the different hydrocarbons, the crude oil mixture must be passed through a fractionating column (also called a distillation column), and is first heated to vaporise the substances into a gas. This separating technique works as each of the chemicals has a specific boiling point, and once each gas has cooled to its specific boiling point - they will condense back into a liquid and can be collected.
Each mixture that is collected from fractional distillation, is called a fraction. A fraction is a mixture of similar length hydrocarbon chains, with similar boiling points.
Many useful materials on which modern life depends are produced by the petrochemical industry, such as solvents, lubricants, polymers, detergents.
A common exam question asks about the steps involved in separating crude oil into its fractions. Let's take a look:
- Crude oil is heated as it enters the fractionating column, and is vaporised
- The column is cooler at the top, and hotter at the bottom
- The gases will begin to rise and as they do so they will cool, and will condense once they reach their boiling point
- The longer the carbon chain, the higher the boiling point - these will condense near the bottom of the column
- The shorter the carbon chain, the lower the boiling point - these will condense near the top of the column
- The different molecules will condense into fractions (mixtures of hydrocarbons with similar carbon chain lengths, and similar boiling points)
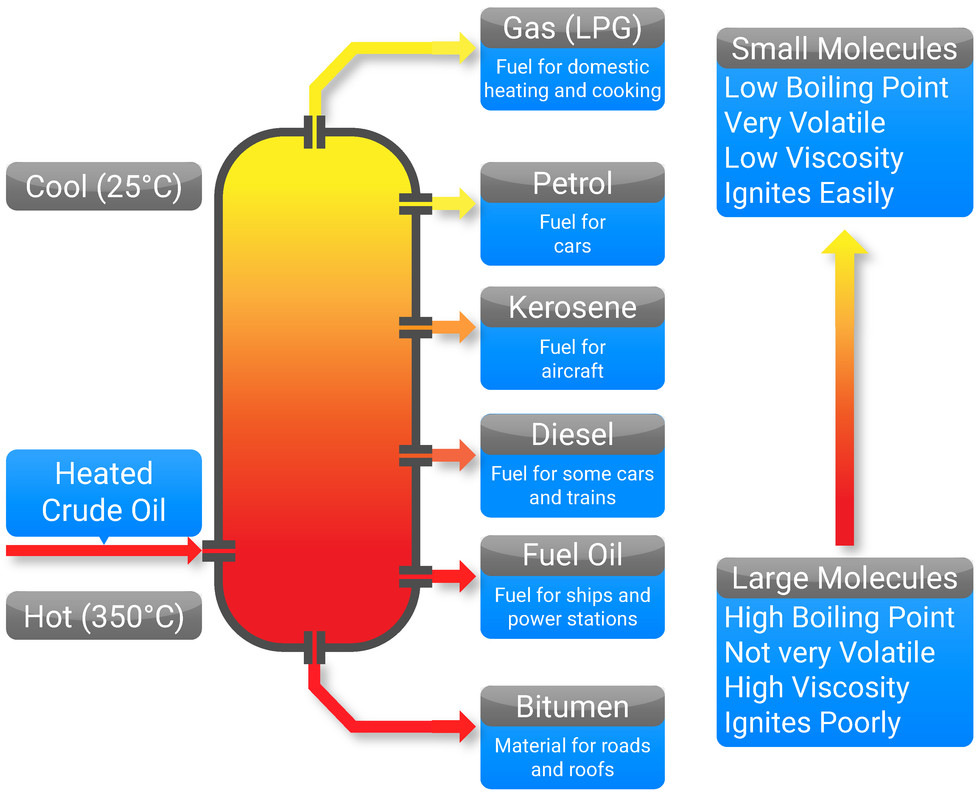
- gases, used in domestic heating and cooking
- petrol (gasoline), used as fuel for cars
- kerosene, used as fuel for aircraft
- diesel oil, used as fuel for some cars and trains
- fuel oil, used as fuel for large ships and power stations
- bitumen, used to surface roads and roofs
Homologous Series: Alkanes
A homologous series is a group of compounds that:
- have the same general formula
- differ by CH2 in molecular formulae from neighbouring compounds
- show a gradual variation in physical properties (e.g. boiling
- have similar chemical properties
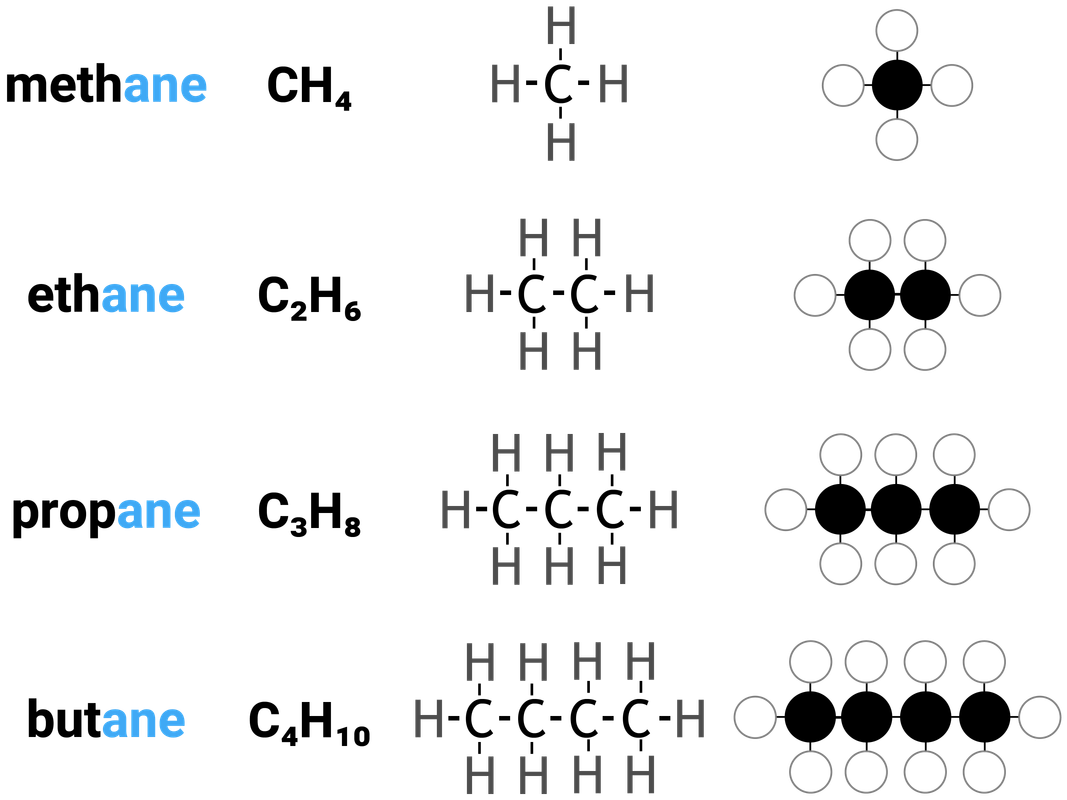
Alkanes are one example of a homologous series. They:
- have the general formula of CnH2n+2
-
this means that for every 1 carbon atom in an alkane, there are two times the amount of hydrogens... plus another two
- differ by CH2 in molecular formulae from neighbouring compounds
-
increasing the carbon chain length by 1 carbon atom, also increases the number of hydrogens by 2
- show a gradual variation in physical properties
-
boiling points increase with increased carbon chain length
viscosity increases with increased carbon chain length
- have similar chemical properties
Because alkanes only contain single bonds between atoms, we describe them as saturated compounds. Think of when a sponge cannot hold any more water, it is "full". A saturated molecule can't fit any more single bonds into it. Alkanes can be considered "full".
Petrol, kerosene and diesel oil are non-renewable fossil fuels obtained from crude oil. They are all mixtures of alkanes.
Methane is a non-renewable fossil fuel found in natural gas.
Combustion
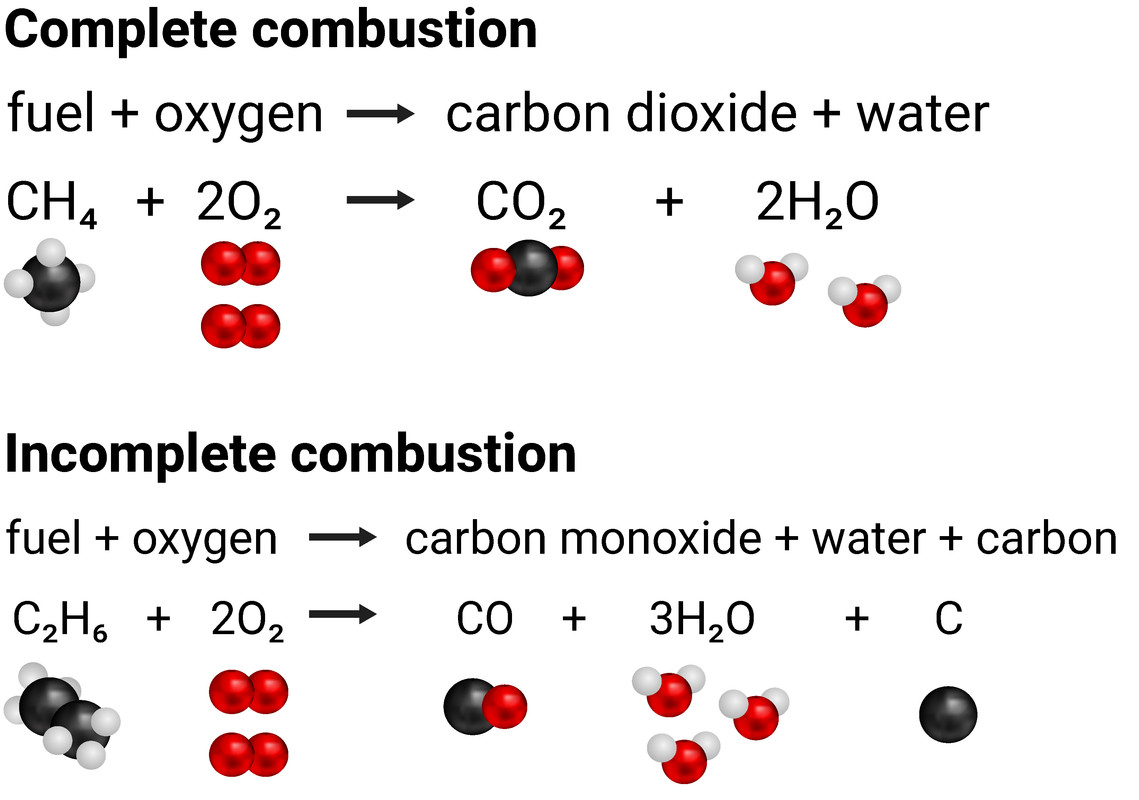
When fuels burn they are reacting with the oxygen in the air. If there is an abundance of air, then complete combustion will take place. This means carbon dioxide and water are produced. If there isn't enough oxygen in the air available, then incomplete combustion happens and carbon monoxide and water are made. In both cases, we can say the carbon (and hydrogen) has been oxidised. Both of these types of combustion are exothermic processes, releasing energy.
Complete combustion
fuel + oxygen (abundant) → carbon dioxide + water
Incomplete combustion
fuel + oxygen (low levels) → carbon monoxide + water (+ carbon particulates)
Incomplete combustion produces carbon monoxide, and carbon particulates, because there is not enough oxygen to fully form carbon dioxide.
Cracking
Some of the heavier hydrocarbons removed during fractional distillation aren't very useful. They are thick liquids with have very high boiling points, and they don't burn very easily.
Cracking involves the breaking down of larger, saturated hydrocarbon molecules (alkanes) into smaller, more useful ones: an shorter saturated alkane, and an unsaturated alkene.
long chain alkane → shorter chain alkane + alkene
The process of cracking is a thermal decomposition. Large chain hydrocarbons are heated, and are passed over a hot catalyst (containing aluminium oxide) and heated to about 650℃. This breaks the large chained alkanes into a smaller alkane and alkene molecule.
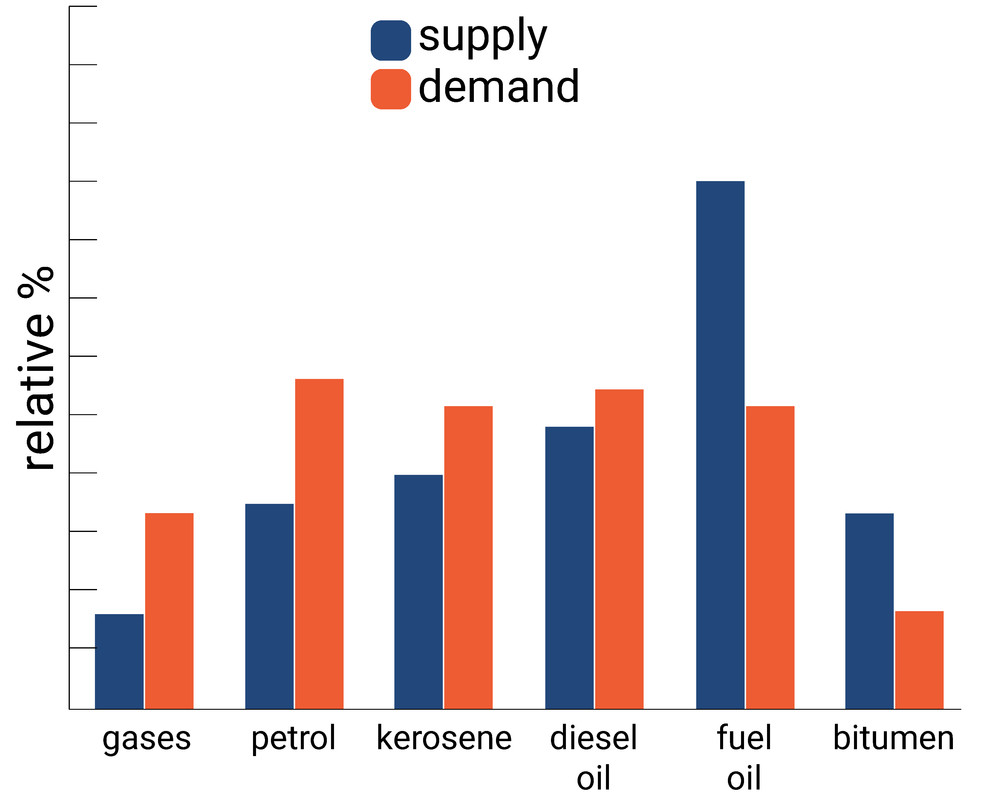
Turning longer chain alkanes into shorter alkanes is extremely beneficial as the current world demand for short alkanes (such as petrol and diesel) is much higher than the supply of these molecules from fractional distillation. The alkenes produced can also be used for making polymers.