
Synthetic and Naturally Occuring Polymers (chemistry only)
Organic Chemistry
Polymers
A polymer is a substance of high average relative formula mass, made up of small repeating units called monomers.
Addition Polymers
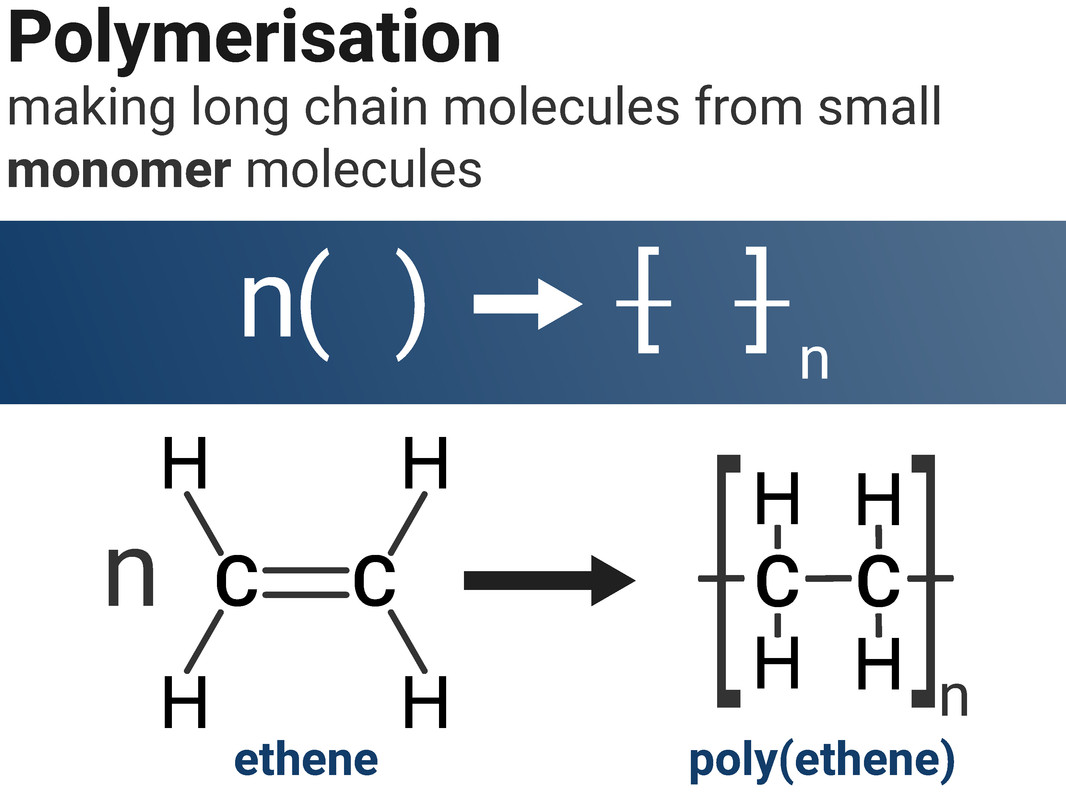
Ethene molecules can combine together in a polymerisation reaction to make poly(ethene). This type of reaction is called addition polymerisation, as we are adding lots of the same monomer together to make one product. The C=C double bond in ethene breaks open to allow ethene molecules to join together.
We don't waste our time drawing out a whole polymer chain, as it contains far too many atoms. Instead, we show the structure of the repeating unit.
When we draw an equation to show an addition polymerisation, it is important that we write n next to both the monomer and the polymer to show large numbers of each are made. We must also draw square brackets around the polymer structure.
Polymers and their Problems
| polymer | properties | uses |
|---|---|---|
| poly(ethene) |
flexible, can be made into thin film | carrier bags, bottles, food wrap |
| poly(propene) |
flexible, strong, shatter-resistant | buckets, bowls, ropes, carpet |
| poly(chloroethene), PVC |
tough, insulator, either hard or flexible | electrical insulation, windows, pipes |
| poly(tetrafluoroethene), PTFE, Teflon™ | slippery, unreactive | non-stick coatings for pans |
There are many problems associated with polymers including the:
- availability of starting materials
- persistence in landfill sites, due to non-biodegradability (microorganisms are unable to break them down)
- greenhouse gases and toxic gases produced during disposal by combustion (to power homes)
- requirement to sort polymers so that they can be melted and reformed into a new product

Recycling polymers is great as it reduces the problems of disposal, and reduces our use of crude oil - but it is difficult and expensive to sort each of the polymers to be recycled.
Biological Polymers
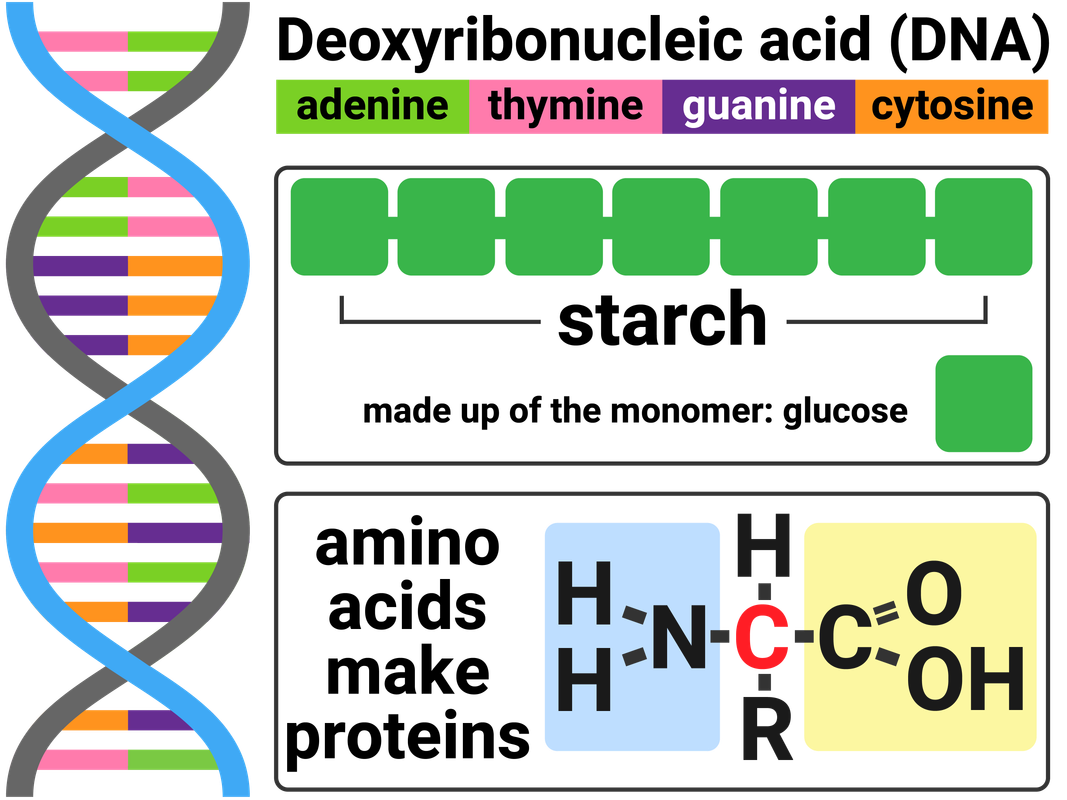
Biological polymers are made naturally by living organisms. DNA encodes genetic instructions for the development and functioning of living organisms and viruses.
Most DNA molecules are two polymer chains, made from four different monomers called nucleotides, in the form of a double helix. These polymer chains join together in the shape of a double helix. Each nucleotide is made of a sugar molecule, a base (A, C, T or G), and a phosphate group.
Carbohydrates include substances such as sugar, starch, and cellulose. These molecules are made from carbon, hydrogen and oxygen. Simple sugars, like glucose, are called monosaccharides. Glucose monomers can join together in long chains to make a polysaccharide called starch.
Higher Tier
Proteins are another example of polymers. There are 21 different amino acids that can be arranged to make proteins we need to survive. Many proteins are enzymes, and/or are essential to our biological functions.
Amino acids have two different functional groups in a molecule. Amino acids react by condensation polymerisation to produce polypeptides.
For example: glycine is H2NCH2COOH and polymerises to produce the polypeptide:
-(-HNCH2COO-)-n and n H2O
Different amino acids can be combined in the same chain to produce proteins.
Condensation Polymers
Higher Tier
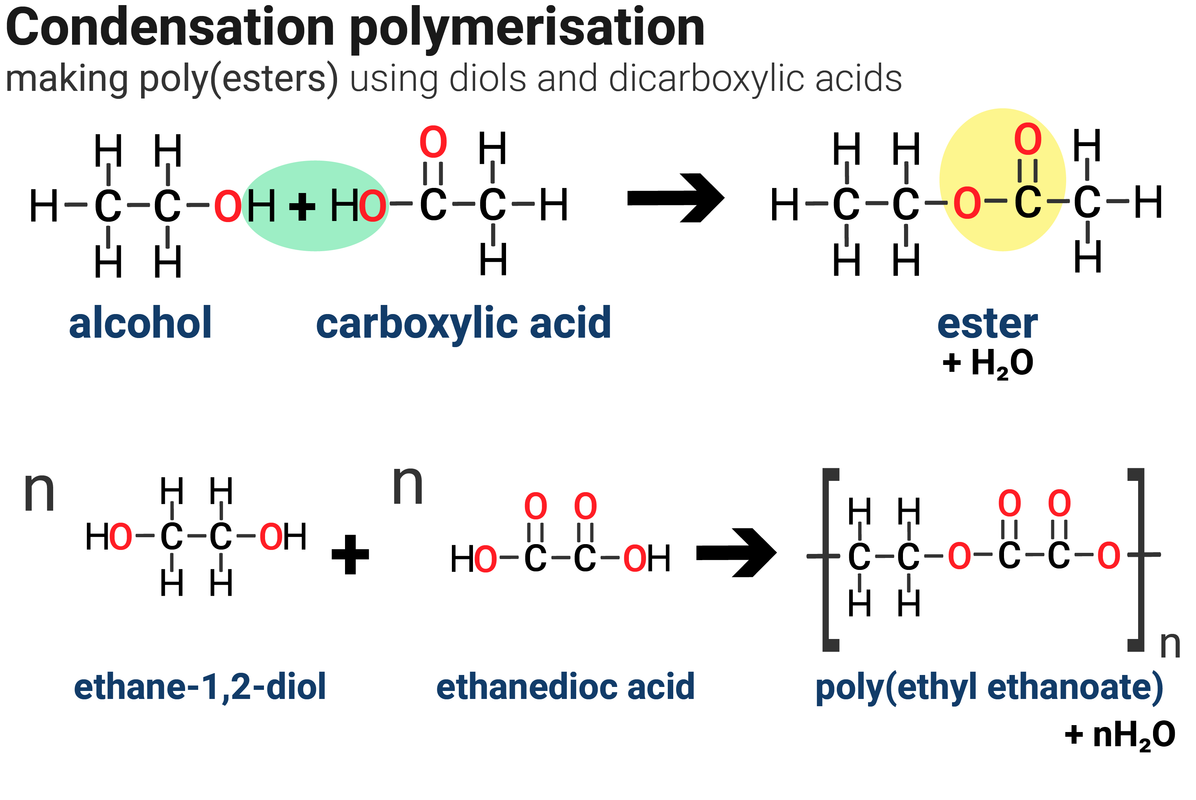
An ester forms when an alcohol reacts with a carboxylic acid. For example:
methanol + ethanoic acid → methyl ethanoate + water
Methyl ethanoate is an ester. For every one ester molecule made, there is a water molecule formed.
Condensation polymerisation is another way of making polymers. Instead of forming just the polymer molecule as the only product, two products form:
- a polymer molecule
- a small molecule, normally water
A polyester requires two different monomers to form a polymer. There must be one of each of the following different monomers:
- a 'dicarboxylic acid' - monomer contains two carboxylic acid groups, –COOH
- a 'diol' - monomer contains two alcohol groups, –OH
Note that:
- both ends of each monomer molecule have a functional group, so can react with another monomer molecule
- one molecule of water is formed every time an ester link is formed