Purity, Formulations, Chromatography and Identification of Common Gases
Chemical Analysis
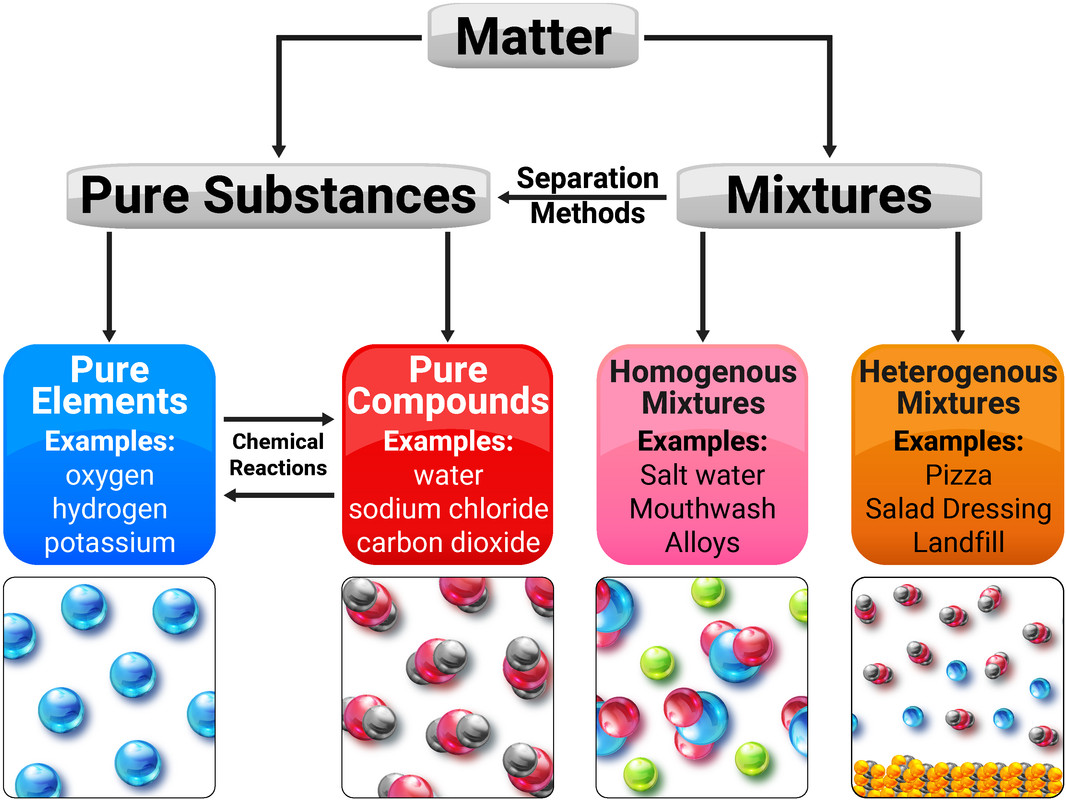
Pure Substances
A pure substance is either a single element, or compound, that is not mixed with any other substance.
A mixture consists of two or more elements/compounds, that are not chemically combined together and can be separated by physical processes.
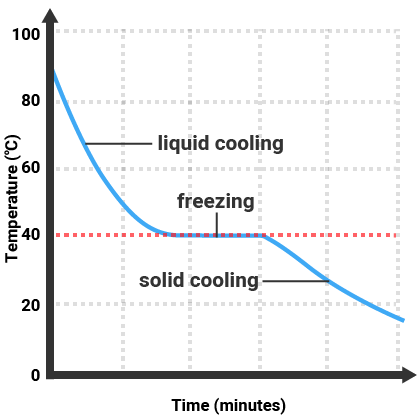
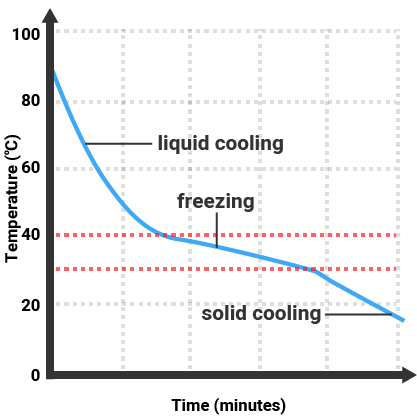
Pure substances will melt and boil at specific temperatures, so we can use the temperature that a substance melts/boils at to tell how pure it is. Impure substances will melt/freeze or boil/condense over a range of temperatures.
 |
 |
| Pure Substance |
Impure Substance |
Formulations
A formulation is a mixture that has been designed as a useful product. Many products are complex mixtures in which each chemical has a particular purpose. Formulations are made by mixing the components in carefully measured quantities to ensure that the product has the required properties. Formulations include fuels, cleaning agents, paints, medicines, alloys, fertilisers and foods.
It is important that formulations keep the same ingredients, so that the formulation is always produced the same way. People buy these products for continuity, especially paints - it would be pointless buying the same shade of paint that was actually different colours.
Fertilisers are formulated so that they add nutrients to the soil for different purposes. NPK fertilisers contain the following chemicals:
- nitrogen (N) is added for plant growth
- phosphorus (P) is added for root growth
- potassium (K) is added to help withstand drought and disease

Chromatography
Chromatography is a method for separating dissolved substances from one another. It works because some of the coloured substances dissolve in the solvent better than others, so they travel further up the paper. This technique is often used to separate out inks, or food colourings.
Paper chromatography consists of two stages:
- the stationary phase (a.k.a. the phase that doesn't move - this is the paper)
- the mobile phase (a.k.a. the phase that moves - this is the solvent)
Separation by chromatography produces a chromatogram, and can be used to distinguish between a pure substance (that produces one spot), and a mixture (impure) which produces multiple spots. Different substances will move at different rates through the paper.
When setting up the experiment you must make sure to draw the baseline in pencil, otherwise the ink from a pen would also dissolve in the solvent.
The Rf value of a spot can be used to identify unknown chemicals if they can be compared to a range of reference substances. The Rf value will always be the same for each substance (when the same solvent is used), and an Rf value will always have a value of less than 1. It can be calculated by using:
Rf value = distance moved by substance ÷ distance moved by solvent
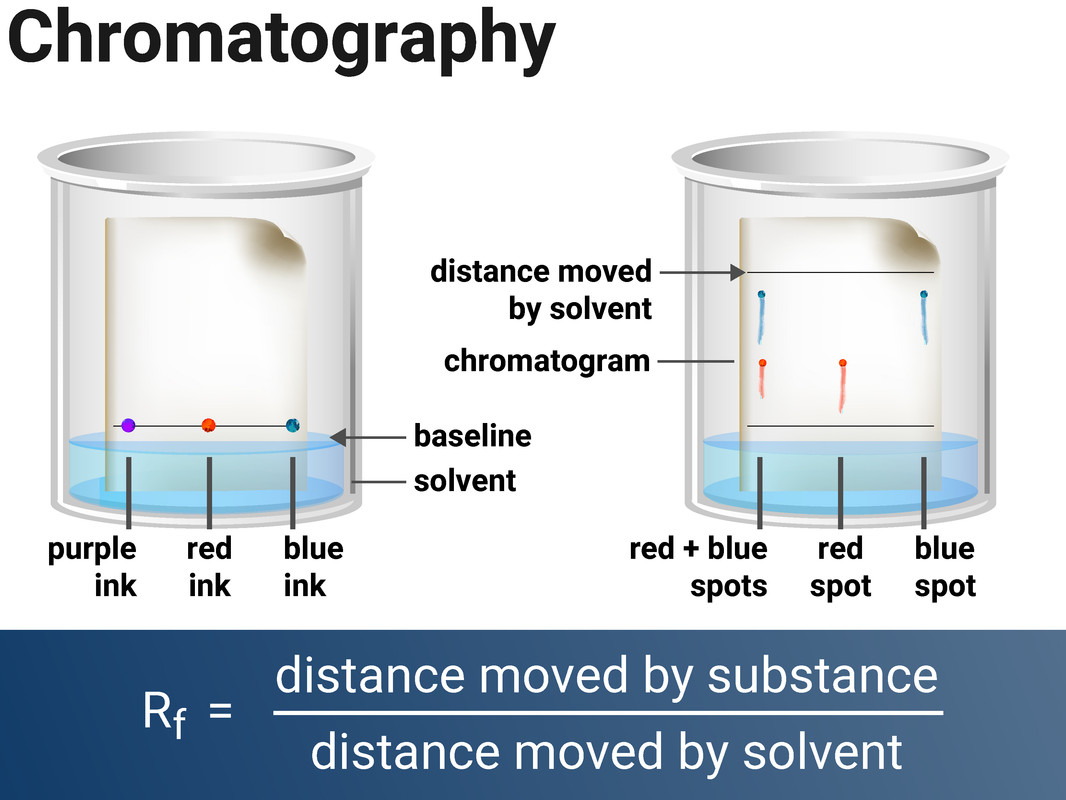
When setting up the experiment you must make sure to draw the baseline in pencil, otherwise the ink from a pen would also dissolve in the solvent.
Test for Gases
Hydrogen
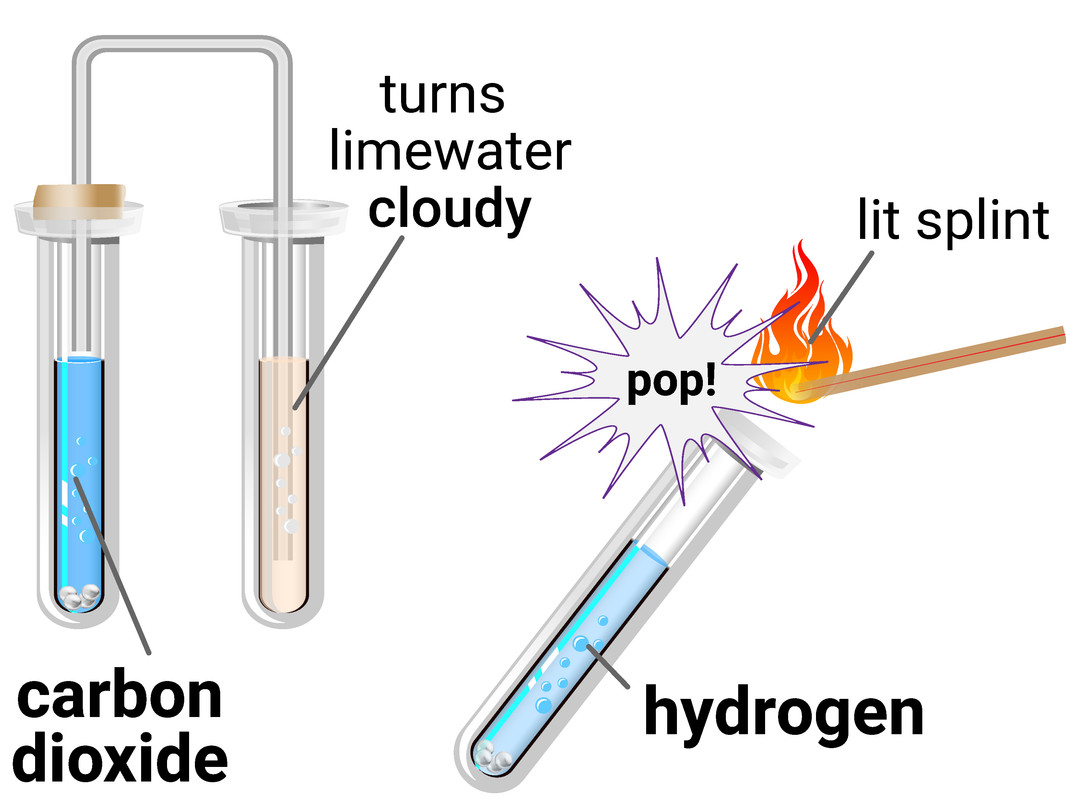
The test for hydrogen uses a burning splint held at the open end of a test tube of the gas. Hydrogen burns rapidly with a pop sound.
This test requires you to allow a second, or two, for oxygen gas from the air to mix with the hydrogen first, as the test is actually showing the chemical reaction between hydrogen and oxygen to make water.
Carbon Dioxide
The test for carbon dioxide uses an aqueous solution of calcium hydroxide (limewater). When carbon dioxide is shaken with or bubbled through limewater the limewater turns milky (cloudy).
This test is actually a reaction between the carbon dioxide and limewater, to make limestone (calcium carbonate).
Oxygen
The test for oxygen uses a glowing splint inserted into a test tube of the gas. The splint relights in oxygen.
Chlorine
The test for chlorine uses litmus paper. When damp litmus paper is put into chlorine gas the litmus paper is bleached and turns white.