Interpreting and interacting with Earth systems
C6: Global challenges
Earth's Atmosphere
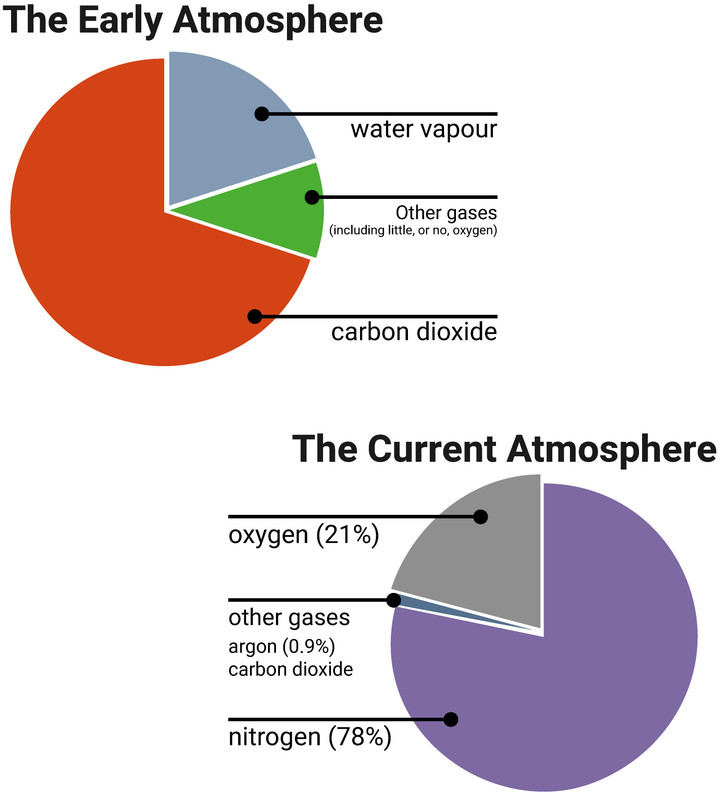
The early atmosphere
It is thought that it was the volcanic activity of a young Earth that produced gases that formed the Earth's early atmosphere. The early atmosphere is thought to have been similar to that of Mars or Venus today, with a large amount of carbon dioxide, small amounts of other gases like ammonia and methane, and little, or no, oxygen. There was also a lot of water vapour in the air, which eventually condensed to form oceans when the Earth cooled.
Over time the amount of carbon dioxide in the air was reduced. One way was by the gas dissolving in newly formed oceans, forming carbonates. These then precipitated into sediments, or sea creatures used the dissolved carbon dioxide to form shells made of calcium carbonate.
Another way that the levels of carbon dioxide decreased was due to the growth of algae and primitive plants that used this carbon dioxide for photosynthesis, which consequently produced oxygen - gradually increasing the amount of oxygen in the atmosphere.
carbon dioxide + water → glucose + oxygen
6CO2 + 6H2O → C6H12O6 + 6O2
Over a few billion years plants evolved and the percentage of oxygen increased to a level that enabled animals to evolve. This nearly killed all other life on the planet, because of how reactive oxygen can be. This is referred to as The Great Oxygenation event.
Testing for oxygen
The test for oxygen uses a glowing splint, which is inserted into a container of the gas. If the flame reignites, then oxygen is present.
Combustion
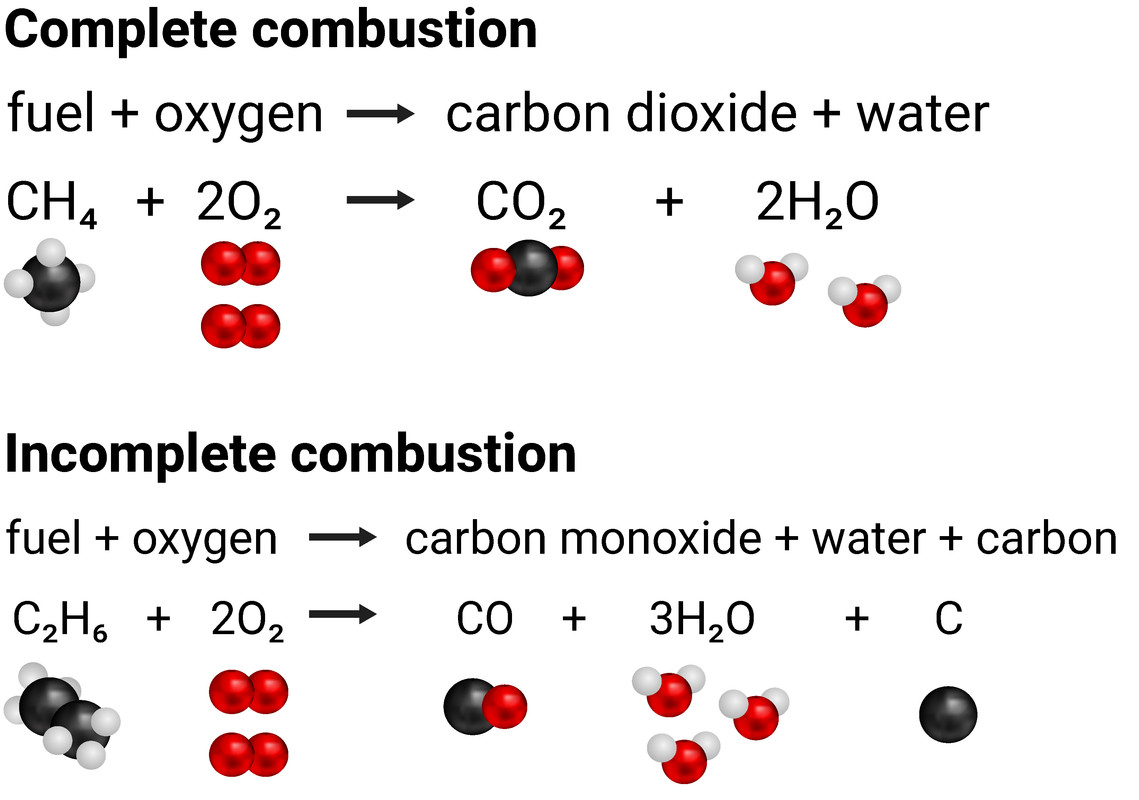
When fuels burn they are reacting with the oxygen in the air. If there is an abundance of air, then complete combustion will take place. This means carbon dioxide and water are produced. If there isn't enough oxygen in the air available, then incomplete combustion happens and carbon monoxide and water are made. In both cases, we can say the carbon (and hydrogen) has been oxidised. Both of these types of combustion are exothermic processes, releasing energy.
Complete combustion
fuel + oxygen (abundant) → carbon dioxide + water
Incomplete combustion
fuel + oxygen (low levels) → carbon monoxide + water (+ carbon particulates)
Incomplete combustion produces carbon monoxide, and carbon particulates, because there is not enough oxygen to fully form carbon dioxide.
Pollutants From Fuels
The combustion of fuels is a major source of atmospheric pollutants.
- carbon monoxide (CO)
-
This is a toxic invisible gas, and it preferentially binds to haemoglobin in red blood cells instead of oxygen. This reduces the amount of oxygen carried in the bloodstream, causing affected people to feel sleepy or to become unconscious. Severe poisoning can even cause death.
- carbon particulates (soot, C)
-
This can block pipes carrying away waste gases from an appliance. It will blacken buildings, and it can cause breathing problems if it collects in the lungs.
Particulates can also contribute to global dimming, reducing the amount of sunlight that reaches the Earth's surface. - sulfur dioxide (SO2)
-
Sulfur impurities in fossil fuels, like coal and crude oil, can react with oxygen when the fuels are burned to produce sulfur dioxide gas. This gas can dissolve in rain water to produce acid rain, which damages structures, and wildlife habitats such as lakes and forests.
- nitrogen oxides (NOx)
-
When fuels are burned in engines, oxygen and nitrogen from the air can react together at high temperatures to produce oxides of nitrogen. These can also dissolve in rain water to produce acid rain.
Nitrogen dioxide is a red-brown toxic gas, and can cause respiratory diseases like bronchitis. Catalytic converters in cars convert most of the NOx gases back to harmless nitrogen.
Some oxides of nitrogen react with other pollutants to form harmful 'photochemical smog'.
Greenhouse Effect
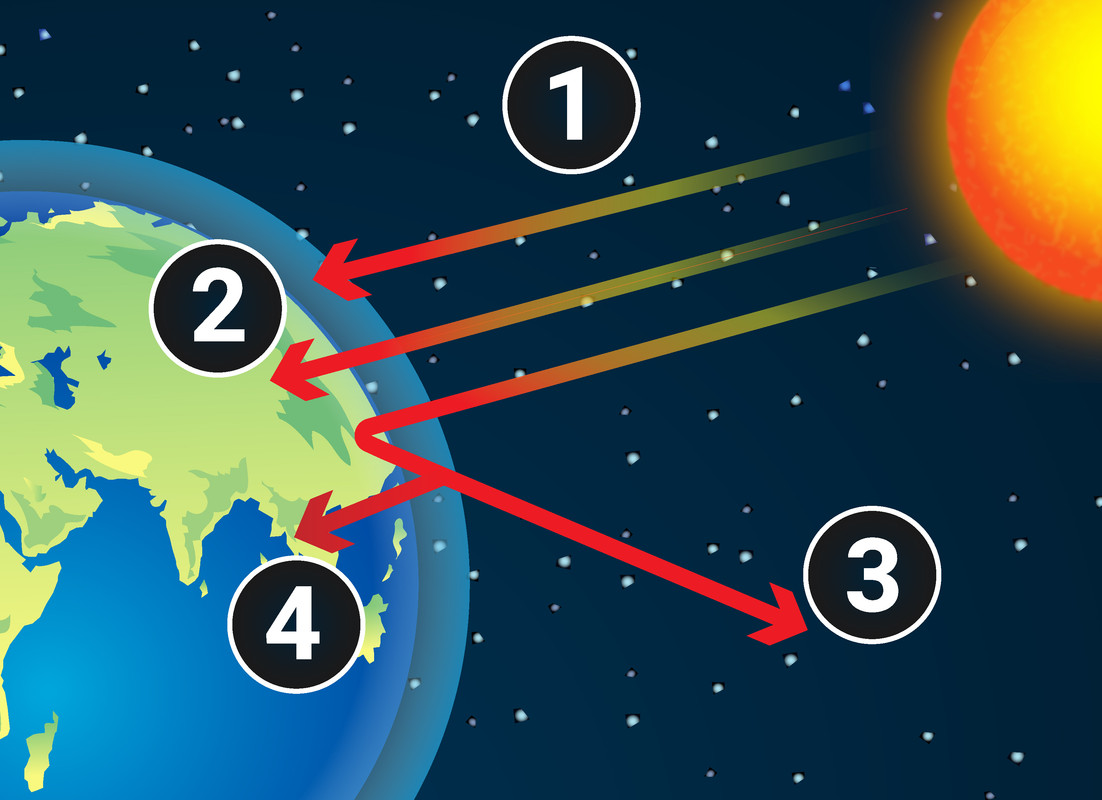
Greenhouse gases in the atmosphere maintain temperatures on Earth high enough to support life. Water vapour, carbon dioxide and methane are all greenhouse gases. They absorb heat radiated from the Earth, subsequently releasing energy which keeps the Earth warm: this is known as the greenhouse effect
- Energy from the Sun is transferred to the Earth by waves, such as light and infrared radiation
- Some energy is absorbed by the Earth's surface, warming it up
- The warm Earth emits (gives out) infrared waves
- Some gases in the air absorb energy transferred by these infrared waves, then re-emit the energy - some of it goes back to the Earth's surface and warms it
Greenhouse gases:
- carbon dioxide (CO2)
- methane (CH4)
- water (H2O)
Anthropogenic (Human Activities) Causes of Climate Change
Based on peer-reviewed evidence, many scientists believe that human activities (releasing lots of greenhouse gases) will cause the temperature of the Earth’s atmosphere to increase resulting in global climate change. Modelling this is difficult, because climate change is a complex issue. This leads to simplified models, speculation, and opinions that are expressed in the media that may be based on only parts of the evidence and can be biased.
Since about 1850, there has been an increase in the burning of fossil fuels for energy and industry. During this time the world's carbon dioxide levels have been increasing, and since we know burning fossil fuels releases carbon dioxide - this is very good evidence that the two are related.
As the levels of carbon dioxide have increased, the average temperature of the Earth's surface has also risen. Whilst there is a strong correlation between the two, correlation doesn't necessarily mean there is a causal link (one thing causes the other) between the two.
Scientists have been able to show in the lab that carbon dioxide absorbs infrared ratiation, and satellites have been able to show that as carbon dioxide levels have increased - less infrared radiation has left the Earth's atmosphere.
Evaluating the evidence
Evidence for historical carbon dioxide levels come from measuring the concentrations of gases trapped in ice cores, the oldest coming from Antartica (with data going back 800,000 years).
The oldest temperature records for central England date back to 1659, but cannot be used to assess global temperature changes as they are only from one place. Temperature records from around the world have only existed from about 1880. These measurements were also not very accurate.
Climate Change
Methane is a much more powerful (some scientists say up to four times more) greenhouse gas than carbon dioxide, as it is better at absorbing infrared radiation. Methane is the main component of natural gas, and is released into the atmosphere when oil and natural gas are extracted from the ground and then processed.
Livestock (mainly cattle) also produce a lot of methane gas because of the bacteria in their stomachs during digestion. There are similar bacteria found in landfill sites, as well as rice fields.
Effects of climate change
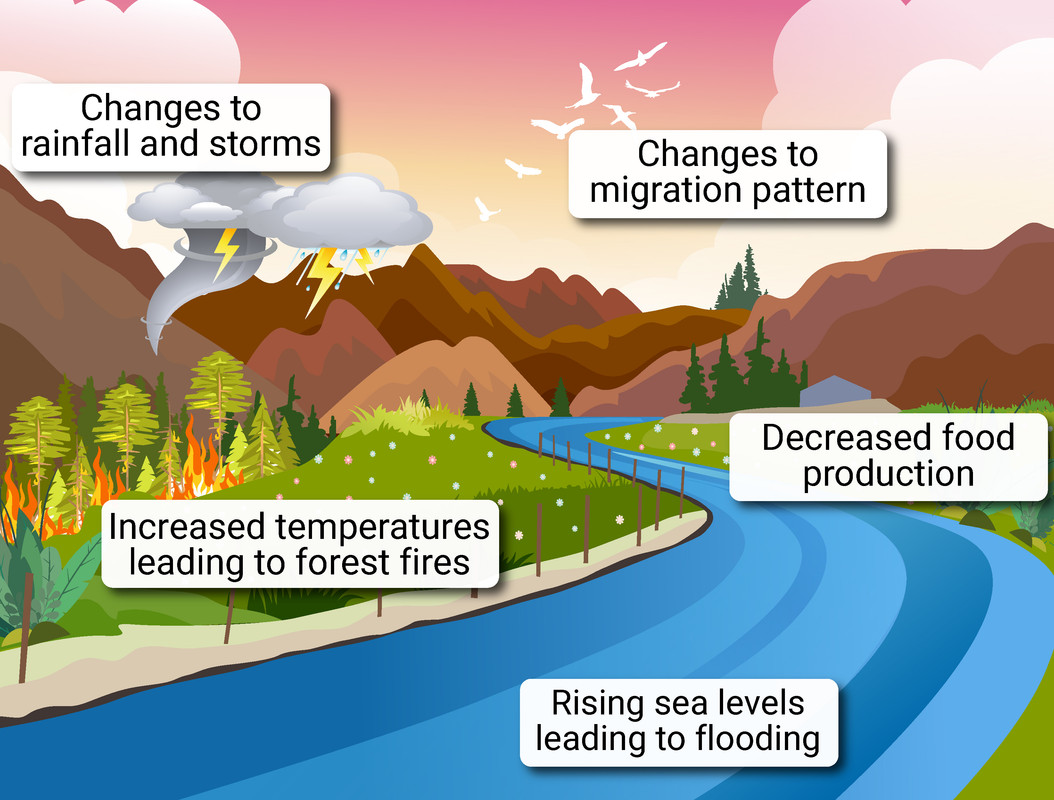
Rising temperatures due to the increase of carbon dioxide and methane in the atmosphere will lead to:
- ice at the South Pole and in glaciers will melt, causing global sea levels to rise - this will lead to increased, and/or permanent flooding in some areas
- animals will move away from their 'natural' habitats to find cooler areas to live, and some plants/animals may become extinct if they cannot survive the warmer temperatures
- weather patterns will begin to change with some areas becoming drier, and others much wetter - and even more extreme weather events like more frequent severe storms and heat waves/droughts
- wildlife, and growth of crops, will be affected by the ever-changing weather systems
- as more carbon dioxide is released, more will dissolve into water systems lowering the pH - more acidic waters can harm the organisms that live in them
Limiting the impact
Using renewable energy sources can reduce greenhouse gas emissions, although it may not undo the damage already done.
Some people have suggested either collecting carbon dioxide and burying it, or reflecting infrared radiation back into space.
Reducing the impact is probably only a temporary fix, but involves building flood defences, dams, and irrigation systems. These will probably destroy habitats, and will not fix the issues - only cover up the symptoms of a much bigger problem.
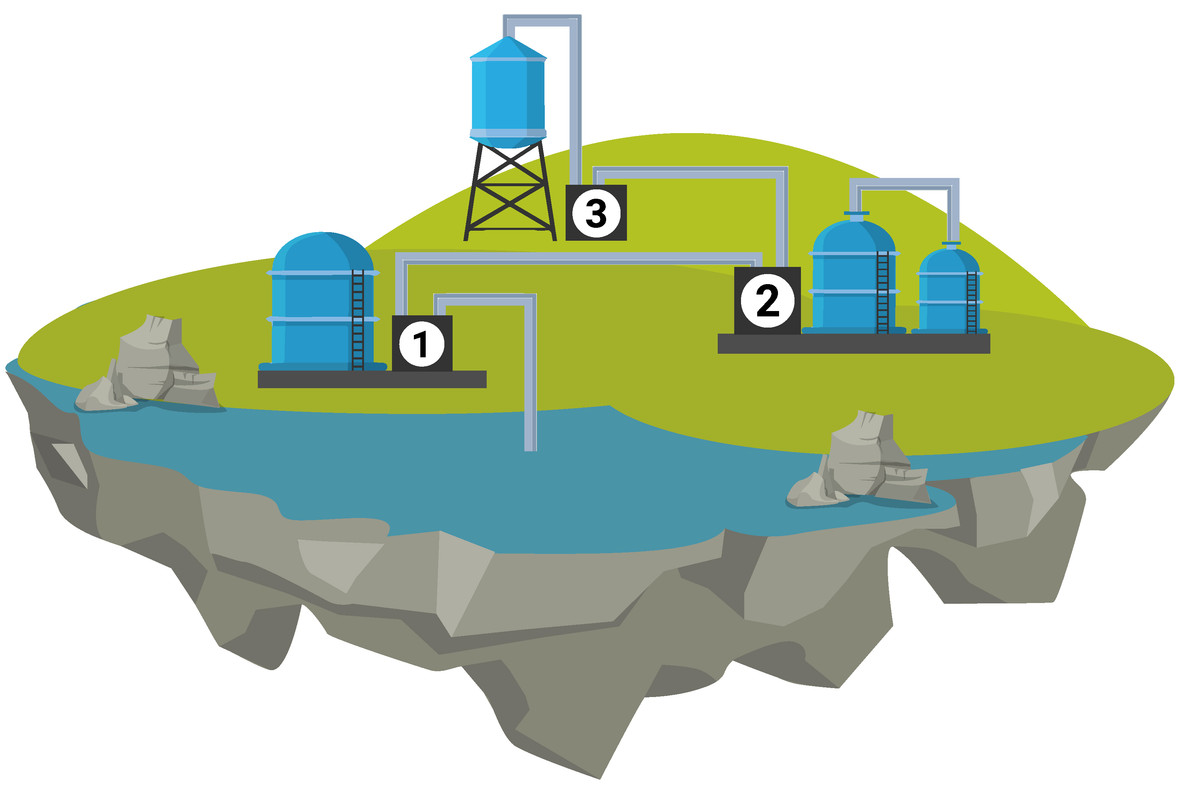
Potable Water
All life requires water to survive. The water they need has to be of good enough quality, which for humans means it needs low levels of dissolved salts and microbes. Water that is safe to drink is called potable water. Potable water is not pure water, because it contains dissolved substances.
There are many methods used to produce potable water, and which method is used will depend on the available supplies of water and local conditions.
In the United Kingdom (UK), rain provides water with low levels of dissolved substances (fresh water) that collects in the ground and in lakes and rivers. To make this water potable, it must go through these steps:
- sedimentation - solids sink to the bottom and are removed
- filtration - fine particles like sand are removed
- chlorination - microbes are killed using chlorine
If supplies of fresh water are limited (such as on an small island), desalination of sea water (high salt levels) may be required. Desalination can be done by distillation. This process requires large amounts of energy and is costly to maintain.
When we do analysis in the lab, we normally use distilled (often called deionised) water. This is because tap water contains dissolved salts that may interfer in our readings, so we must use pure water as these salts have been removed.