
Organic chemistry (chemistry only)
C6: Global challenges
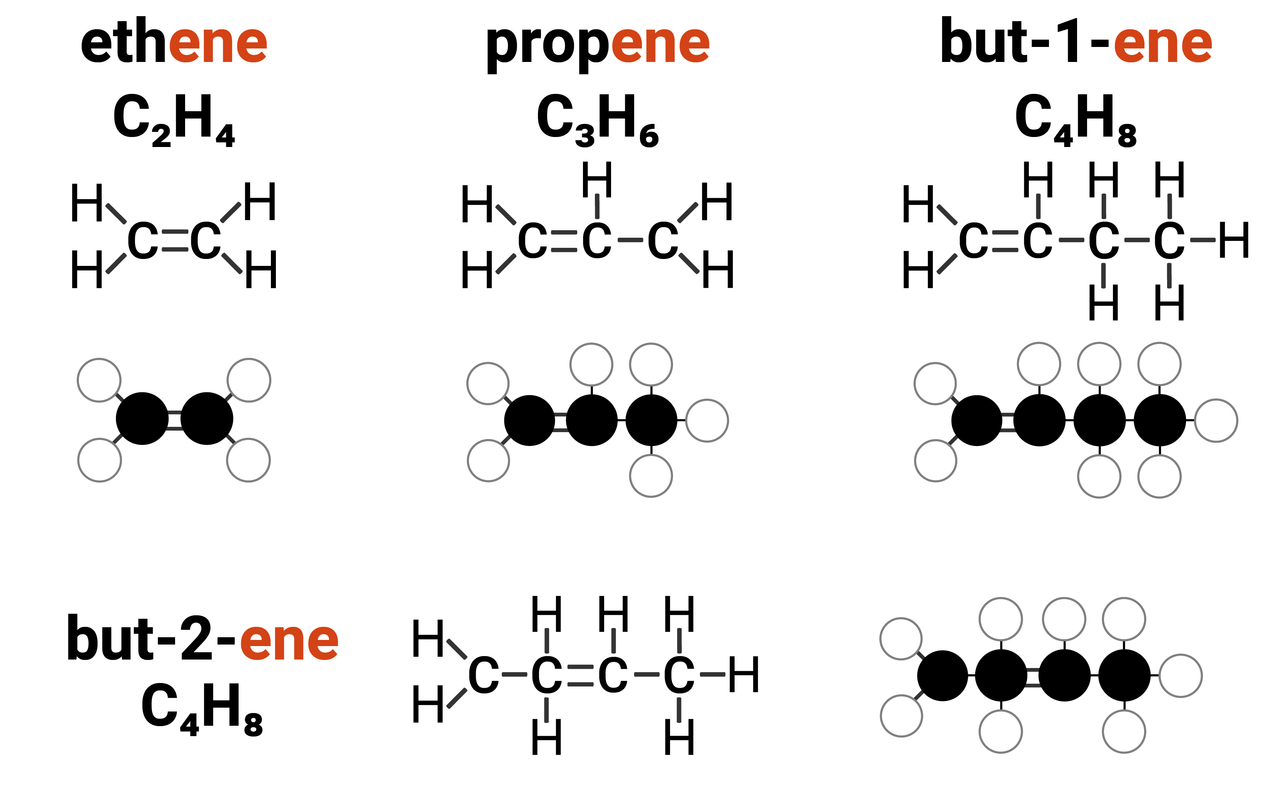
Homologous Series: Alkenes
Alkenes are one example of a homologous series. They:
- have the general formula of CnH2n
-
this means that for every 1 carbon atom in an alkane, there are two times the amount of hydrogens
- differ by CH2 in molecular formulae from neighbouring compounds
-
increasing the carbon chain length by 1 carbon atom, also increases the number of hydrogens by 2
- show a gradual variation in physical properties
-
boiling points increase with increased carbon chain length
viscosity increases with increased carbon chain length
- have similar chemical properties
Because alkenes contain at least one double bond between carbon atoms, we describe them as unsaturated compounds. Think of when a sponge cannot hold any more water, it is "full". A saturated molecule can't fit any more single bonds into it. Alkenes can fit more single bonds in (once the double bonds are broken).
Alkenes can have more than one carbon-carbon double bond, however you will only ever be expected to draw an alkene with one of these bonds.
For ethene and propene, the double bond can only go in one place, however for any chains longer than 3 carbons - this can get a bit more complicated. You need to know the structures of all the isomers of butene that can form due to the double bond being in one of two places.
Reactions of Alkenes
Addition reactions
The functional group (double carbon bond, C=C) allows alkenes to undergo addition reactions. The reaction is an 'addition' reaction because one molecule combines with another molecule, forming one larger molecule and no other products.
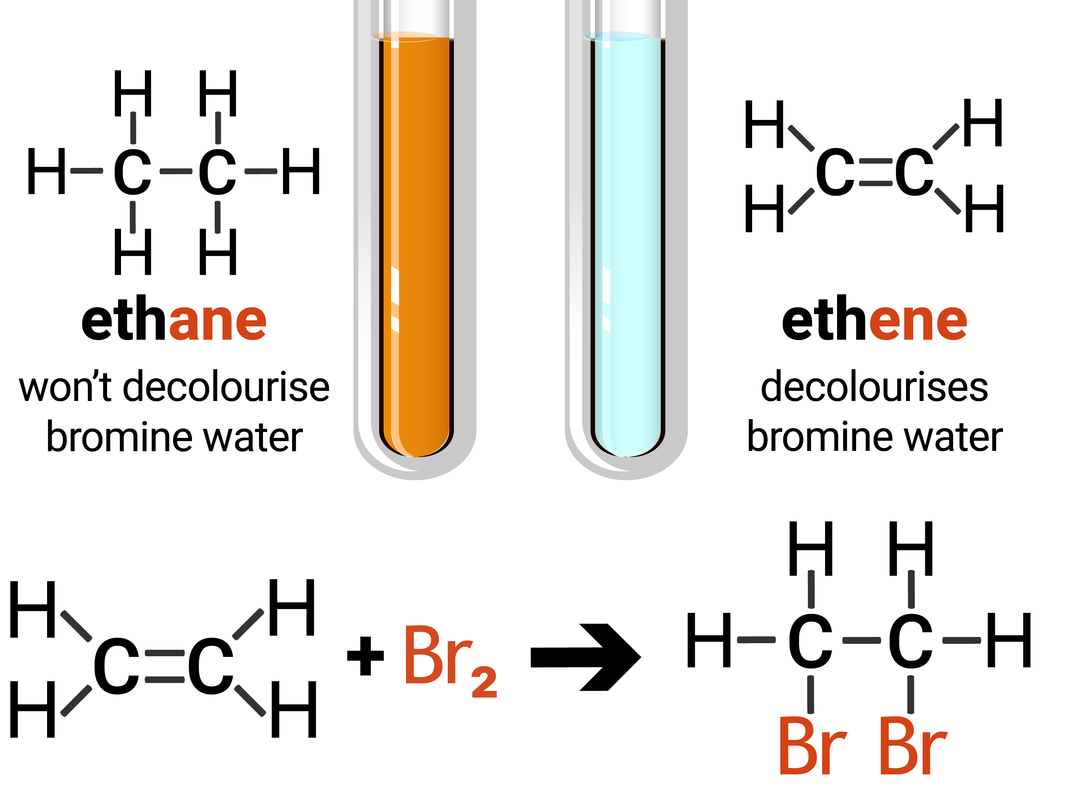
The test for alkenes makes use of the addition reaction, and involves adding bromine water to a sample of hydrocarbon. If an alkane is present the solution stays orange-brown, but an alkene will turn the solution colourless. This is because the bromine is added across the carbon-carbon double bond.
Complete combustion
The complete combustion of alkanes and alkenes involves the oxidation of both the carbon and hydrogen atoms in hydrocarbons, producing carbon dioxide and water. For example:
ethane + oxygen → carbon dioxide + water
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l)
ethene + oxygen → carbon dioxide + water
C2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(l)
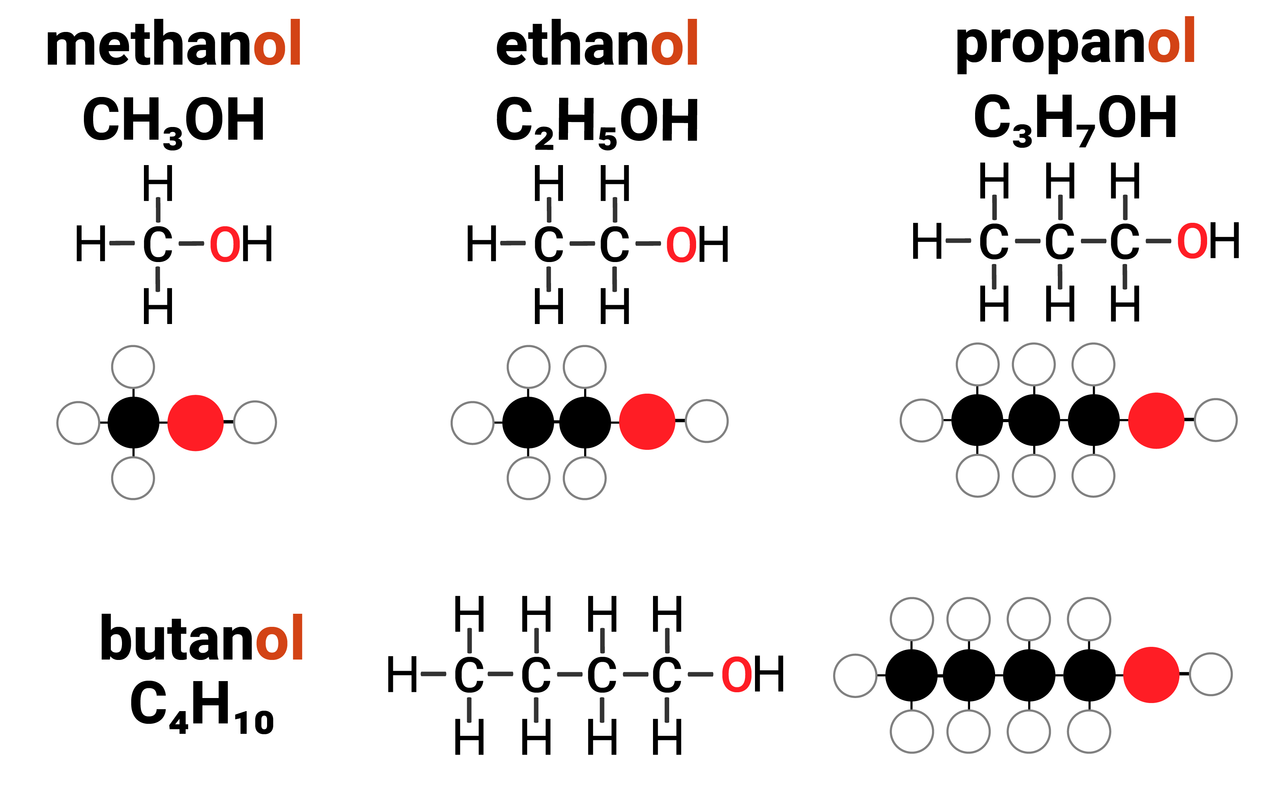
Homologous Series: Alcohols
Alcohols are one example of a homologous series. They:
- have the general formula of CnH2n+1OH
-
this means that for every 1 carbon atom in an alcohol, there are two times the amount of hydrogens... plus one more, and an OH group
- differ by CH2 in molecular formulae from neighbouring compounds
-
increasing the carbon chain length by 1 carbon atom, also increases the number of hydrogens by 2
- show a gradual variation in physical properties
-
boiling points increase with increased carbon chain length
viscosity increases with increased carbon chain length
- have similar chemical properties
Alcohols have the functional group of -OH (hydroxyl) which gives rise to the ending of alcohol. It is responsible for the general reactions that all alcohols can do.
The longer the carbon chain of an alcohol, the more energy the molecule stores. When burned, this energy is released in the form of heat and can be measured using a calorimeter.
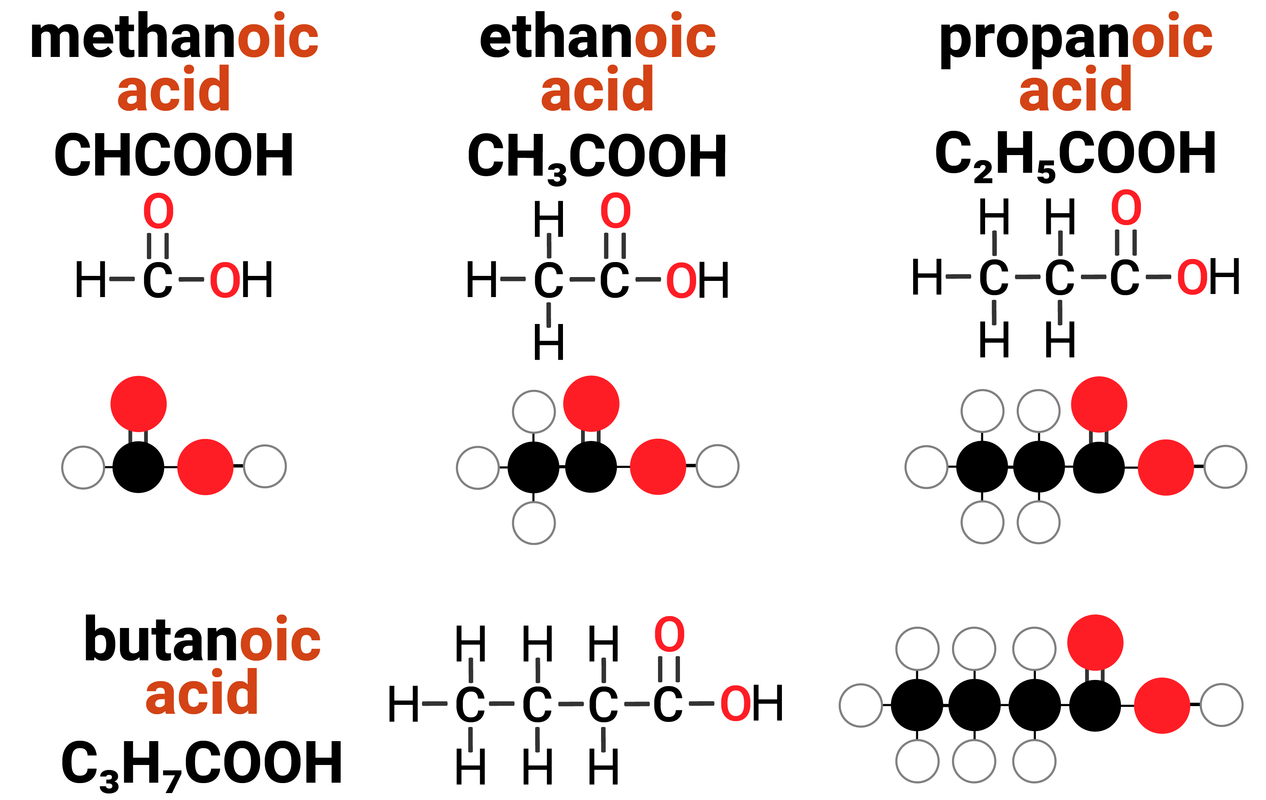
Homologous Series: Carboxylic Acids
Carboxylic acids are one example of a homologous series. They:
- have the general formula of CnH2n+1COOH
-
this means that for every 1 carbon atom in a carboxylic acid, there are two times the amount of hydrogens... plus one more, and a COOH group
- differ by CH2 in molecular formulae from neighbouring compounds
-
increasing the carbon chain length by 1 carbon atom, also increases the number of hydrogens by 2
- show a gradual variation in physical properties
-
boiling points increase with increased carbon chain length
viscosity increases with increased carbon chain length
- have similar chemical properties
Carboxylic acids have the functional group of -COOH (carboxyl) which gives rise to the ending of carboxylic acid. It is responsible for the general reactions that all carboxylic acids can do.
Reactions of Alcohols and Carboxylic Acids
Alcohols
- colourless liquids that dissolve in water to form neutral solutions
- can be dehydrated to form alkenes (produces a water molecule)
- will react with the alkali metals to produce hydrogen and a metal hydroxide (like sodium would with water)
- burn in air to produce carbon dioxide and water (complete combustion)
- can be oxidised to make a carboxylic acid in the presence of an oxidising agent (shown as [O])
Carboxylic acids
- dissolve in water to form acidic solutions (pH values less than 7)
- react with metals to form a salt and hydrogen
- react with bases to form a salt and water
- react with carbonates to form a salt, water and carbon dioxide
Polymers
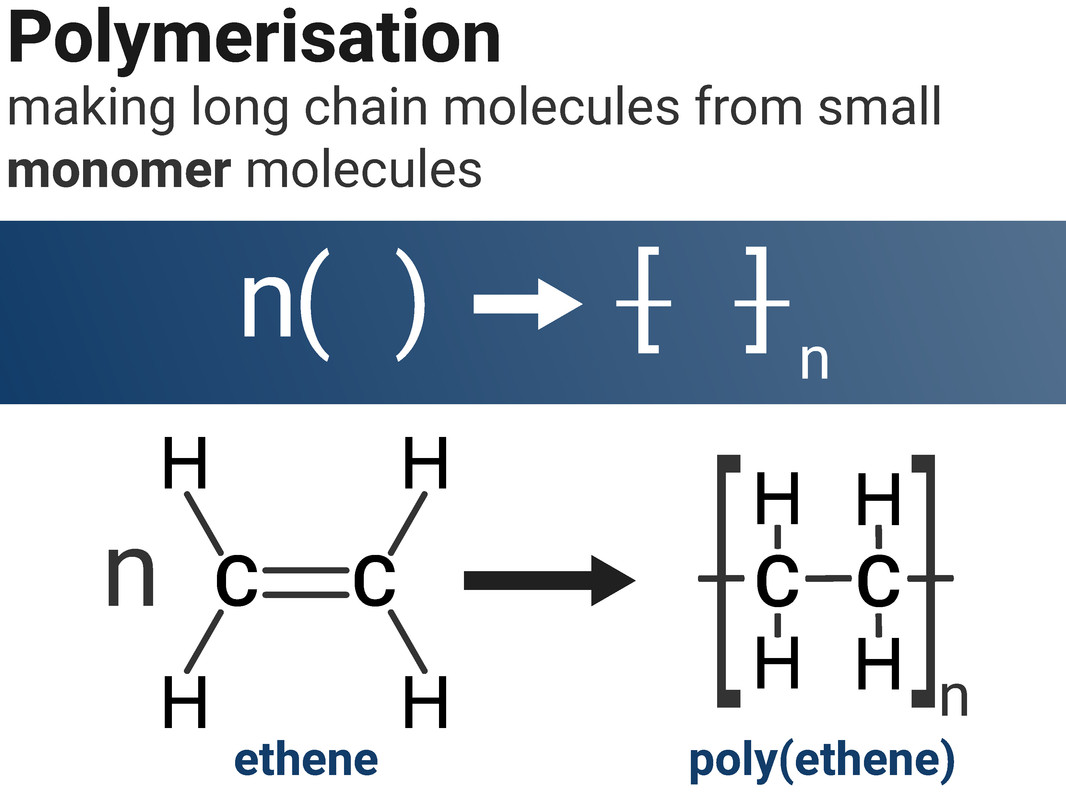
A polymer is a substance of high average relative formula mass, made up of small repeating units called monomers.
Addition Polymers
Ethene molecules can combine together in a polymerisation reaction to make poly(ethene). This type of reaction is called addition polymerisation, as we are adding lots of the same monomer together to make one product. The C=C double bond in ethene breaks open to allow ethene molecules to join together.
We don't waste our time drawing out a whole polymer chain, as it contains far too many atoms. Instead, we show the structure of the repeating unit.
When we draw an equation to show an addition polymerisation, it is important that we write n next to both the monomer and the polymer to show large numbers of each are made. We must also draw square brackets around the polymer structure.
Biological Polymers
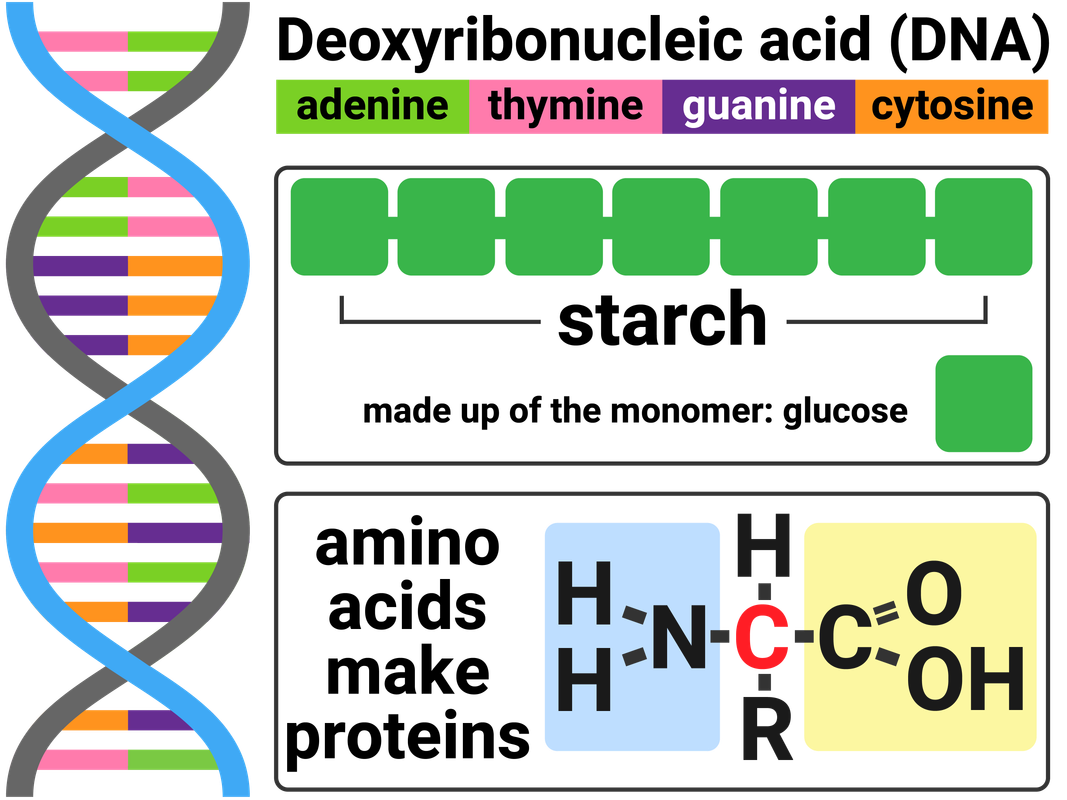
Biological polymers are made naturally by living organisms.
DNA is a polymer made from four different monomers called nucleotides. Most DNA molecules are made from two polymer chains. These polymer chains join together in the shape of a double helix. Each nucleotide is made of a sugar molecule, a base (A, C, T or G), and a phosphate group.
Carbohydrates include substances such as sugar, starch, and cellulose. These molecules are made from carbon, hydrogen and oxygen. Simple sugars, like glucose, are called monosaccharides. Glucose monomers can join together in long chains to make a polysaccharide called starch.
Proteins are another example of polymers. There are 21 different amino acids that can be arranged to make proteins we need to survive. Many proteins are enzymes, and/or are essential to our biological functions.
Higher Tier
Amino acids have two different functional groups in a molecule. Amino acids react by condensation polymerisation to produce polypeptides.
For example: glycine is H2NCH2COOH and polymerises to produce the polypeptide:
-(-HNCH2COO-)-n and n H2O
Different amino acids can be combined in the same chain to produce proteins.
Condensation Polymers
Higher Tier
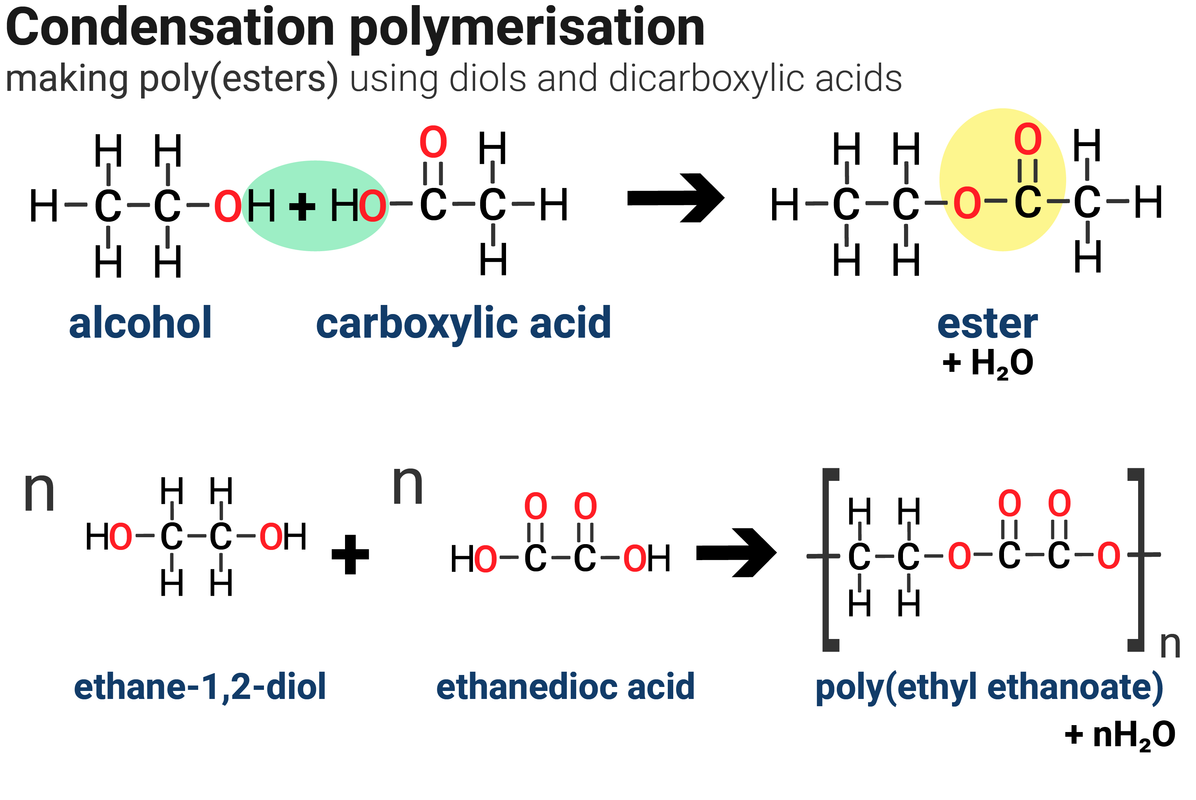
An ester forms when an alcohol reacts with a carboxylic acid. For example:
methanol + ethanoic acid → methyl ethanoate + water
Methyl ethanoate is an ester. For every one ester molecule made, there is a water molecule formed.
Condensation polymerisation is another way of making polymers. Instead of forming just the polymer molecule as the only product, two products form:
- a polymer molecule
- a small molecule, normally water
A polyester requires two different monomers to form a polymer. There must be one of each of the following different monomers:
- a 'dicarboxylic acid' - monomer contains two carboxylic acid groups, –COOH
- a 'diol' - monomer contains two alcohol groups, –OH
Note that:
- both ends of each monomer molecule have a functional group, so can react with another monomer molecule
- one molecule of water is formed every time an ester link is formed
Chemical Cells and Fuel Cells
Chemical cells use chemical reactions to convert and transfer energy to electrical energy. They will produce a voltage only up until one of the reactants has been used up (we say the battery has "gone flat").
A simple chemical cell is made up of two parts:
- two different metals, each dipped into a solution of one of their salts
- a 'salt bridge' to allow dissolved ions to pass from one solution to the other
Batteries, like the ones in torches and mobile phones, are made up of chemical cells.
There are many types of chemical cell, each with different chemical reactions.
Fuel cells will produce a voltage continuously, provided they have a constant supply of fuel and oxygen (from the air).
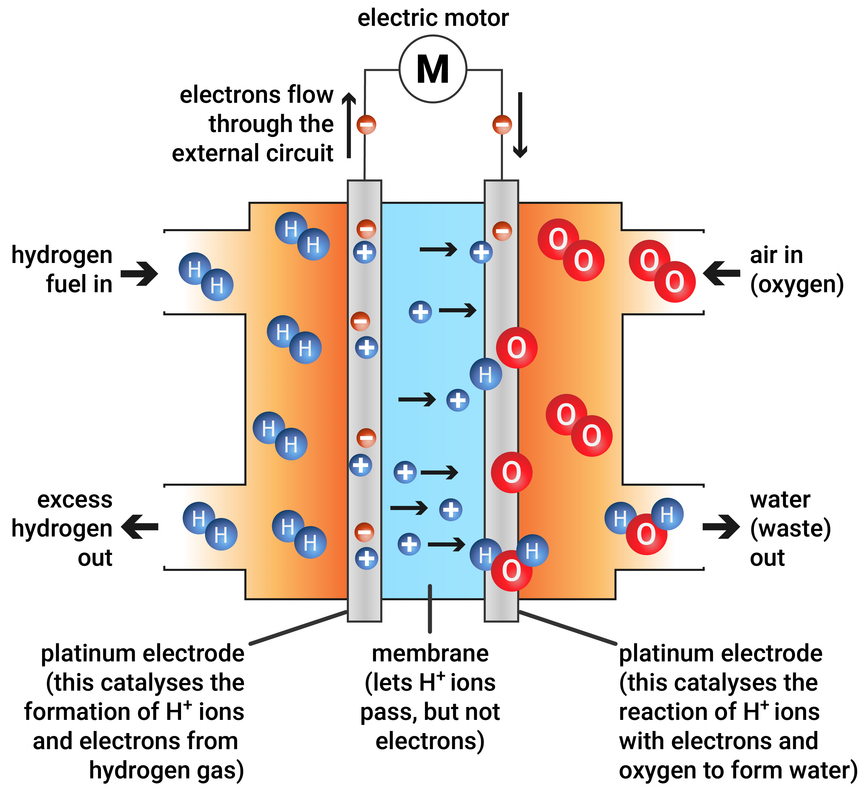
Hydrogen-oxygen fuel cells
In this type of fuel cell, hydrogen and oxygen are used to produce a voltage. The only product from this reaction is water.
A hydrogen-oxygen fuel cell and electric motor are much quieter, and need less maintenance, than a petrol or diesel engine, but the hydrogen still needs to be stored in a container - like a tank.
hydrogen + oxygen → water
2H2 + O2 → 2H2O
Higher Tier
At the cathode: 2H2(g) → 4H+(aq) + 4e-
At the anode: O2(g) + 4H+(aq) + 4e- → 2H2O(l)
Uses of Fuel Cells
Hydrogen, diesel and petrol are all highly flammable fuels. Fuel cells have their advantages and disadvantages depending on the use.
Fuel cells in spacecraft
| strengths | weakness |
|---|---|
| no moving parts to maintain | have to be continuously supplied with oxygen and hydrogen although this could be rectified by using solar cells to electrolyse the water produced back into oxygen and hydrogen |
| small in size for the amount of electricity produced | |
| water they produce can be used for drinking water |
Fuel cells in vehicles
| strengths | weaknesses | |
|---|---|---|
| fuel cells |
|
|
| petrol/diesel |
|
|