
Improving processes and products
C6: Global challenges
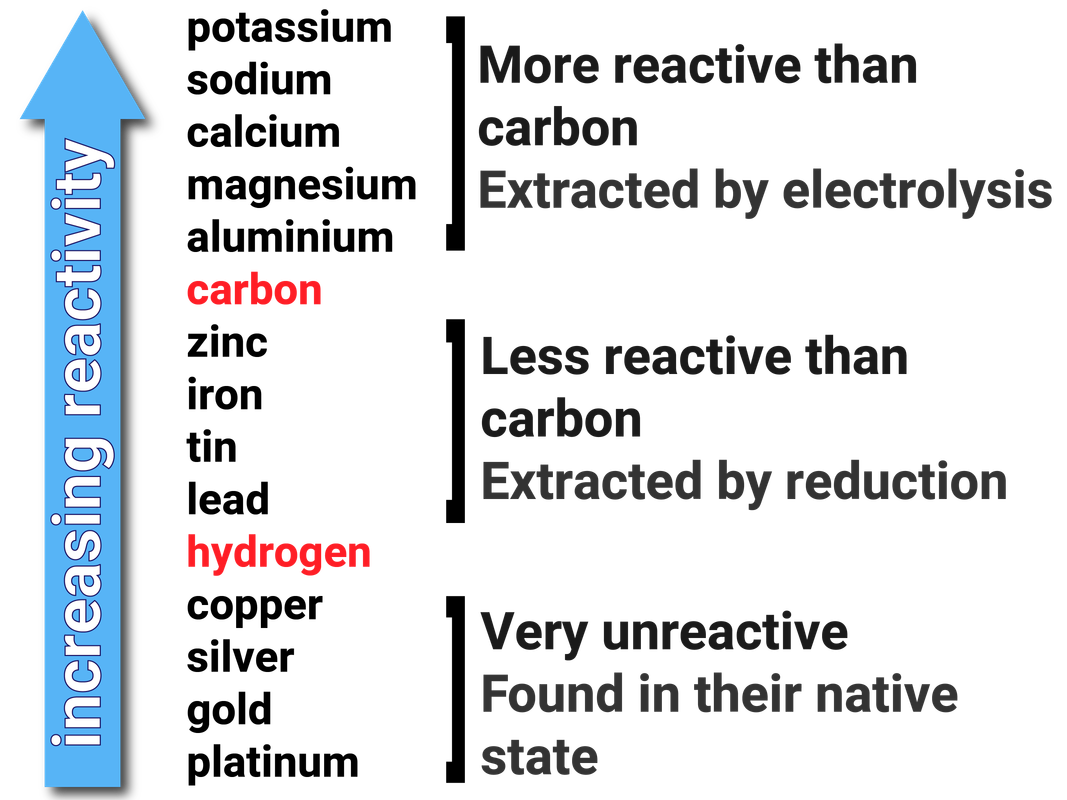
Reactivity Series
When metals react with other substances the metal atoms form positive ions. The reactivity of a metal is related to its tendency (how likely it is) to form positive ions.
Metals can be arranged in order of their reactivity in a reactivity series. The metals potassium, sodium, lithium, calcium, magnesium, zinc, iron and copper can be put in order of their reactivity from their reactions with water and dilute acids.
The non-metals hydrogen and carbon are often included in the reactivity series to give an indication about how the metals can be extracted.
Metals less reactive than hydrogen are generally found in their elemental form in the Earth's crust (native state). They do not need to be extracted.
Metals less reactive than carbon can be extracted by heating the metal with carbon. As the carbon is more reactive, it will displace the metal in its ore - reducing the metal.
Metals more reactive than carbon have to be extracted using electrolysis. This is very costly, in terms of both money and energy.
REDOX (Reduction and Oxidation)
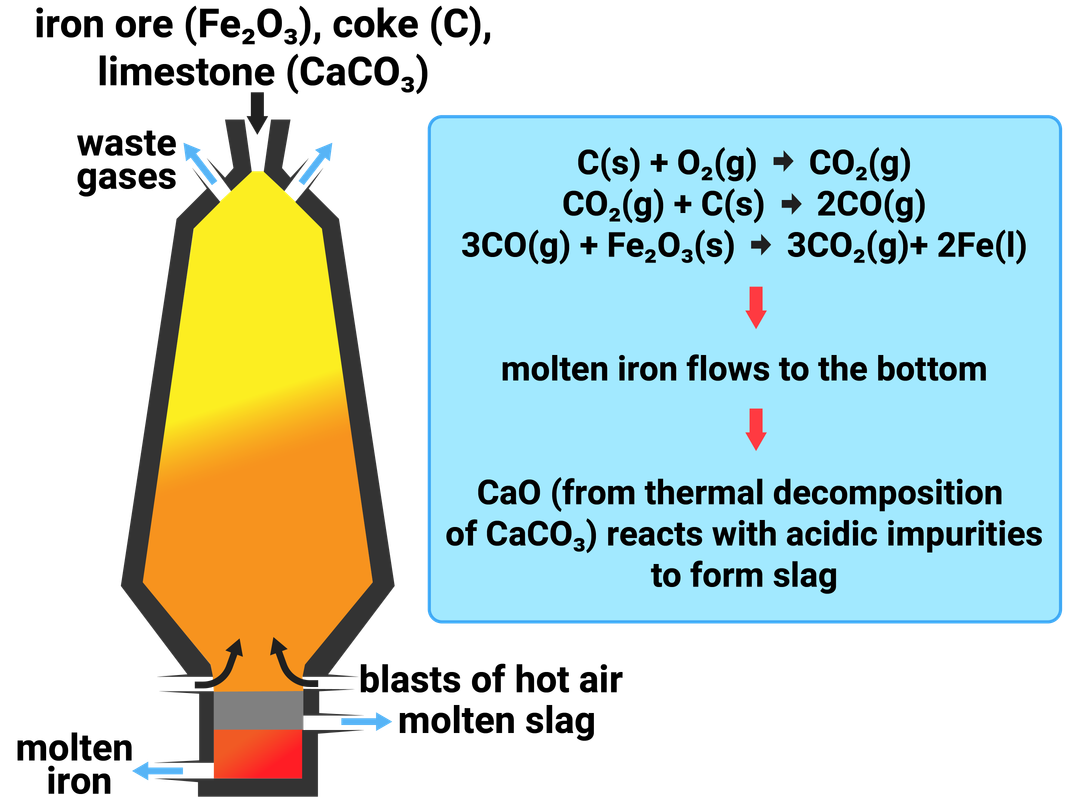
Most metals do not exist naturally on their own, but combined with other elements, and compounds, as ores in the Earth's crust. Most ores contain oxygen, and so when a metal reacts with oxygen a metal oxide is formed. This is called oxidation.
Removing the oxygen from the compound to extract the metal is called reduction. To extract iron we need to reduce the ore. This means we take away the oxygen.
Iron is naturally found as iron oxide, so we can reduce the ore by heating it with a reducing agent. By adding carbon monoxide, we can remove the oxygen from the ore to make iron and carbon dioxide:
iron oxide + carbon monoxide → iron + carbon dioxide
Fe2O3 + CO → Fe + CO2
The metals right at the bottom of the reactivity series are unreactive, and are found in their native state as uncombined elements in the Earth's crust.
Knowledge of the blast furnace (shown here) is not needed, and is only shown to illustrate how carbon is used to extract iron.
Extracting Metals
The least reactive metals are found in their native state in the Earth's crust. This means they are found as uncombined elements.
Any metal more reactive than hydrogen, but less reactive than carbon, can be extracted by adding carbon to displace the metal from its oxide (or other compounds). This is known as reduction.
The most reactive metals have to be extracted using electrolysis, as carbon is not reactive enough to displace the metals. This is rather costly, and uses a lot of electricity.
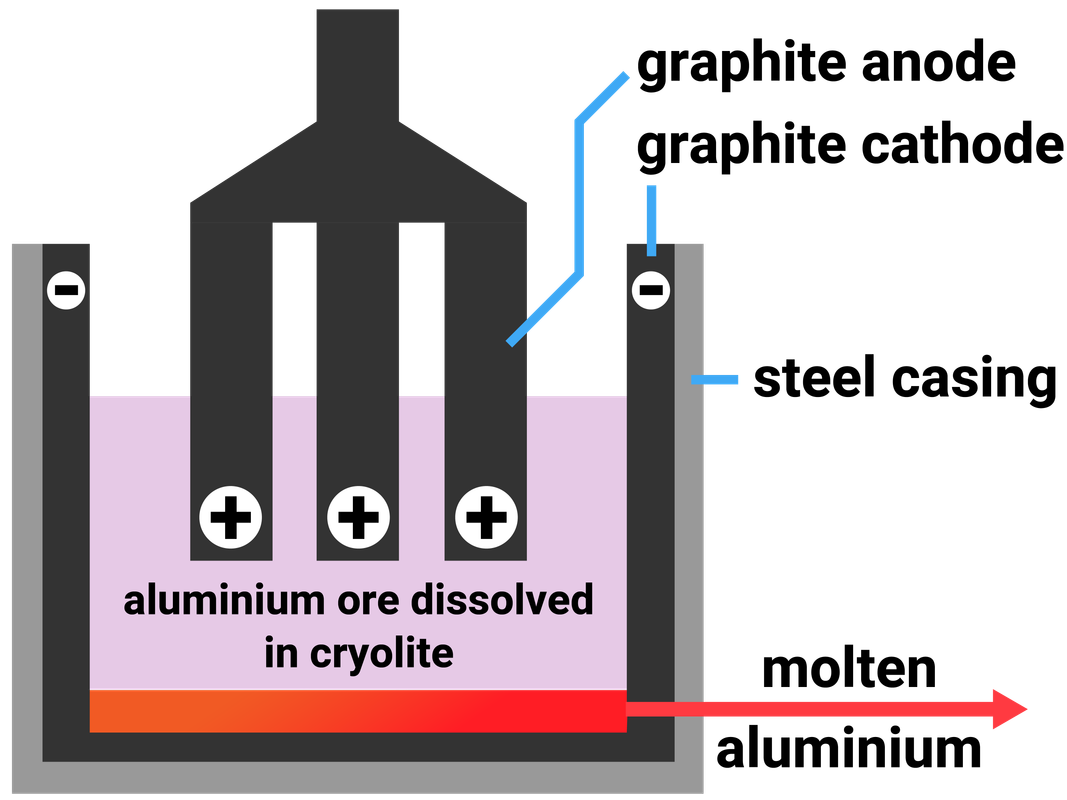
Extracting Aluminium
Aluminium oxide is insoluble in water, so it must be molten to act as an electrolyte. A lot of energy must be transferred to break aluminium's ionic bonds, which is expensive - so to reduce costs, powdered aluminium oxide is dissolved in molten cryolite. This melts at a lower temperature than aluminium oxide and helps reduce costs.
Higher Tier
Half equation at the cathode:
Al3+ + 3e- → Al
Half equation at the anode:
2O2- → O2 + 4e-
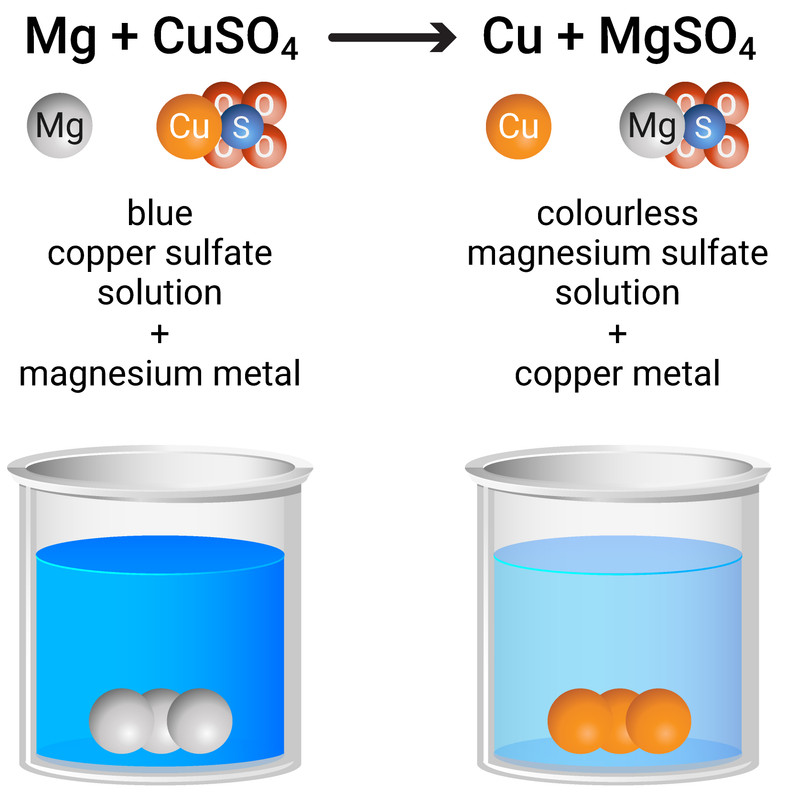
Displacement Reactions
A more reactive metal can displace a less reactive metal from a compound in solution. This is one way to extract metals. For example:
magnesium + copper sulfate → copper + magnesium sulfate
Mg + CuSO4 → Cu + MgSO4
Higher Tier
Ionic equations only show us the ions that change in a chemical reaction. This is useful as it shows what is oxidised and reduced. We can start by writing a balanced symbol equation for a reaction:
Mg(s) + CuSO4(aq) → Cu(s) + MgSO4(aq)
Then we can write out all the ions involved:
Cu²⁺(aq) + SO4²⁻(aq) + Mg(s) → Cu(s) + Mg²⁺(aq) + SO4²⁻(aq)
We can identify the ions that don't change, these are called the spectator ions. These are of no interest in the reaction, so we can ignore them and write out our ionic equation:
Mg(s) + Cu²⁺(aq) → Cu(s) + Mg²⁺(aq)
Once we have an ionic equation, we can take it one step further and look at half equations. These look at how the electrons behave. We can see there are only two different chemicals involved, and we can split our equation in half... to make half equations:
Cu²⁺ + 2e⁻ → Cu
Mg - 2e⁻ → Mg²⁺
By writing our equation in this way, we can immediately see that copper had to gain two electrons (reduced) in this reaction, and those electrons came from magnesium (oxidised).
Life Cycle Assessments
Life cycle assessments (LCAs) are carried out to assess the environmental impact of products at each stage of its 'life'. This process is referred to as a 'cradle to grave' analysis. The stages assessed include:
- extracting and processing raw materials
- using up limited resources
- damaging habitats
- using up limited resources
- manufacturing and packaging
- using up land for factories
- the use of machines and people
- using up land for factories
- use and operation during its lifetime
- cleaning
- repair
- using up limited resources
- disposal at the end of its useful life
- using up land for landfill sites
- is the product recycled or reused
- using up land for landfill sites
We often need to take transport of the raw materials, and the product, into account - as these can be transported huge distances back-and-forth from country to country, via ships or aeroplanes before they even end up at your home, school, or workplace.
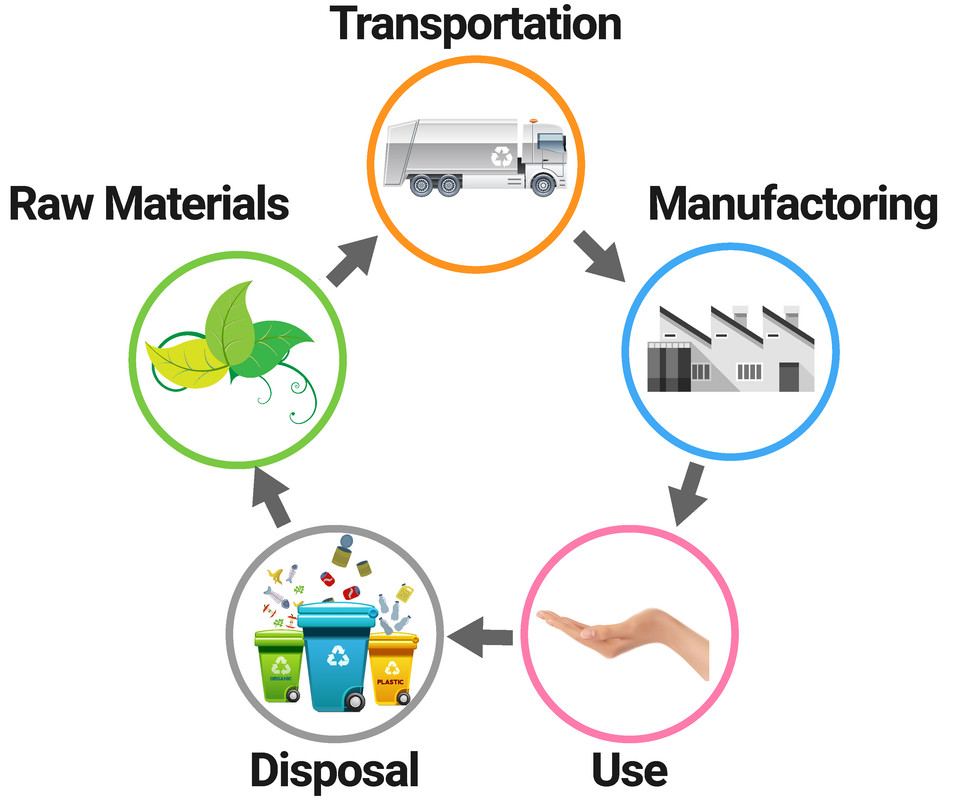
Recycling Materials
Waste materials such as polymers and metals are often disposed of in landfill sites. Waste polymers may be incinerated.
Crude oil and metal ores are two important raw maerials, but they are finite resources - they either form extremely slowly or are no longer being made. Recycling reduces the issues of disposal and conserves our limited raw materials.
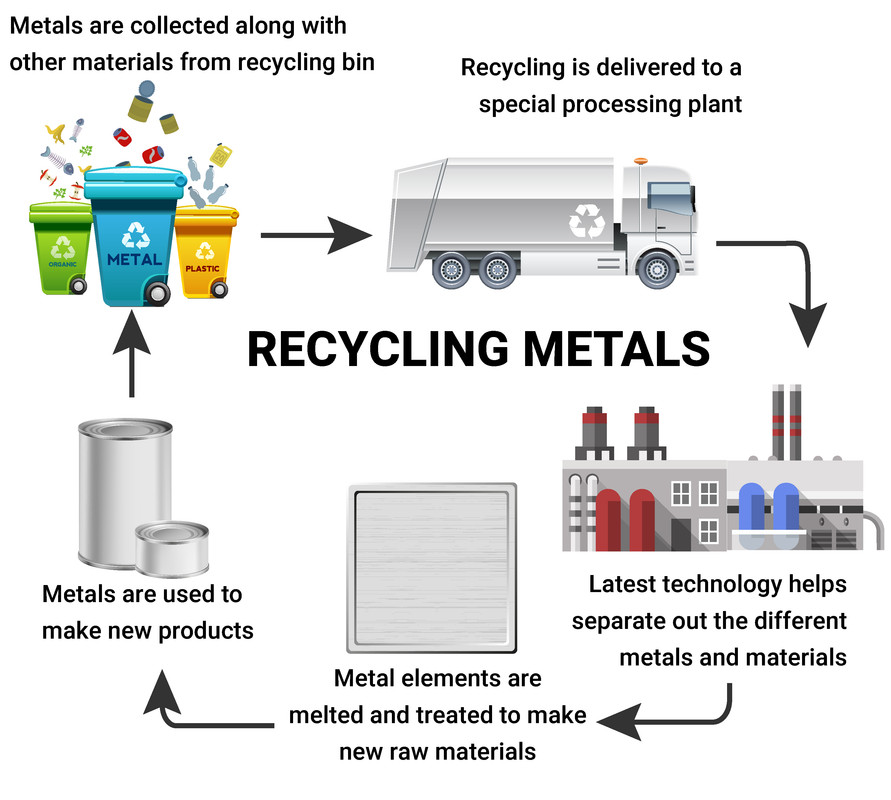
Recycled metals and polymers allow us to save on resources, but first require the metal to be collected. We can make new materials out of these recycled materials. The steps involved are:
- collecting the used material, and taking it to a recyling centre
- sorting the materials and/or breaking them up
- removing any of the impurities, or unwanted items
Polymers then need to be melted and reformed into different products.
Metals need to be also need to be melted, but are often then solidifyed into ingots. The ingots can then be used to manufacture new metal items.
Some of the main advantages are:
- natural reserves will last longer
- less mining of ores will mean the landscape is less damaged, also reducing noise and dust pollution
- fewer oil wells, quarries or mines are needed
- less energy is generally required to recycle metals than extract them from ores
- less waste metal will end up in landfill
- smaller areas of natural habitats are damaged
Disadvantages of recycling :
- the collection and transport of used items needs organisation, workers, vehicles and fuel
- it can be difficult to sort different materials from one another
- the sorted materials may need to be transported to where they can be processed
Alternative, Biological Methods of Extraction
Higher Tier
The Earth’s resources of metal ores are limited. One of the metals we are worried about is copper, as its ores are becoming scarce. Luckily new ways of extracting copper from low-grade ores have been developed. These include phytoextraction, and bioleaching.
No harmful gases are produced (e.g. sulfur dioxide) like in traditional mining, both methods cause less damage to the landscape than traditional mining, and both methods conserve supplies of high grade ores.
The main issue with these processes is that they're both very slow.
Photoextraction
Plants absorb mineral ions through their roots, and this method of extraction makes use of this:
- plants are grown on a low-grade ore (contains less metal)
- plants absorb metal ions through their roots
- plants are harvested and burnt
- ash left behind contains a higher concentration of the metal than the original ore
- ash is then processed to obtain the metal
Phytoextraction is more expensive than mining some ores. Growing plants is also dependent on weather condition. It does, however, allow us to extract metals from contaminated soils.
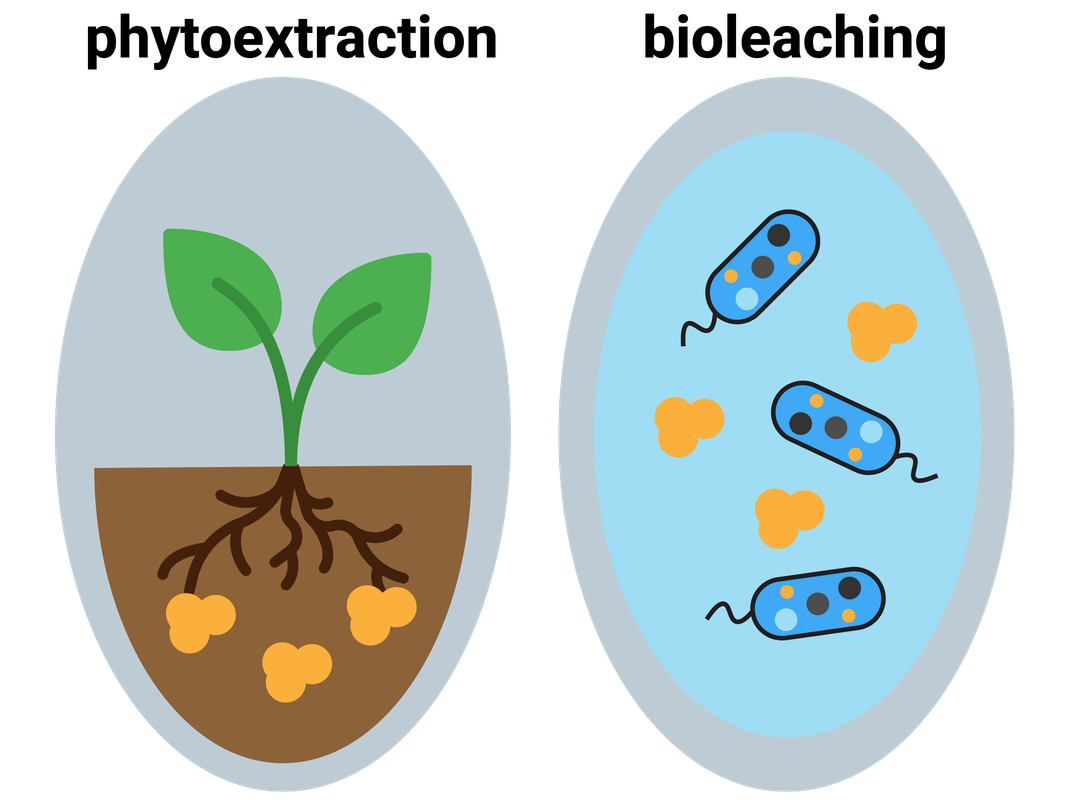
Bioleaching (bacterial extraction)
Bacteria can break down low-grade ores to produce an acidic solution (called a leachate) containing metal ions. Copper, nickel, cobalt and zinc ions can be extracted this way, and they are then displaced using scrap iron with a final purification step of electrolysis.
It doesn't need high temperatures, but it does produce toxic substances and sulfuric acid which damage the environment.