
Electrolytic Processes
Chemical Changes
Electrolysis
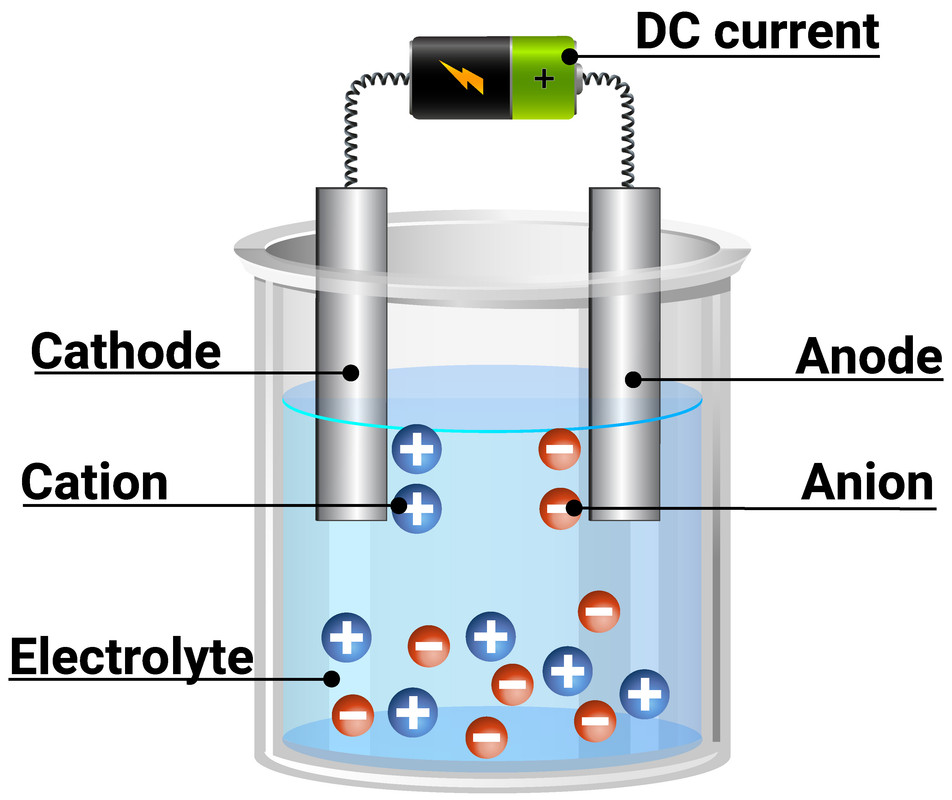
Electrolysis is the process of passing electrical current (direct current) through a solution or molten ionic compound, to decompose electrolytes. This separates the ions, and elements will be discharged at the electrodes. Inert electrodes should be used, otherwise they will react with the solution/products.
Electrolysis can only be done using ionic compounds, as it requires free ions to move. This is why it can only be done if in solution, or liquid (molten) state. If electrolysis is completed with a solution, then hydrogen (H+) and hydroxide (OH-) ions will also be present, as water will also dissociate.
During electrolysis:
- positively charged ions (cations) migrate to the negatively charged electrode (cathode)
- negatively charged ions (anions) migrate to the positively charged electrode (anode)
Rules of electrolysis
- at the cathode (negative electrode) - the least reactive element (metal or hydrogen) will be discharged
- at the anode (positive electrode) - in order of preference:
- halide ions (F⁻, Cl⁻, Br⁻ etc.) will be discharged as halogen gases
- hydroxide ions (OH⁻) - given off as oxygen gas
- any other negative ions present
- halide ions (F⁻, Cl⁻, Br⁻ etc.) will be discharged as halogen gases
Electrolysis of Solution
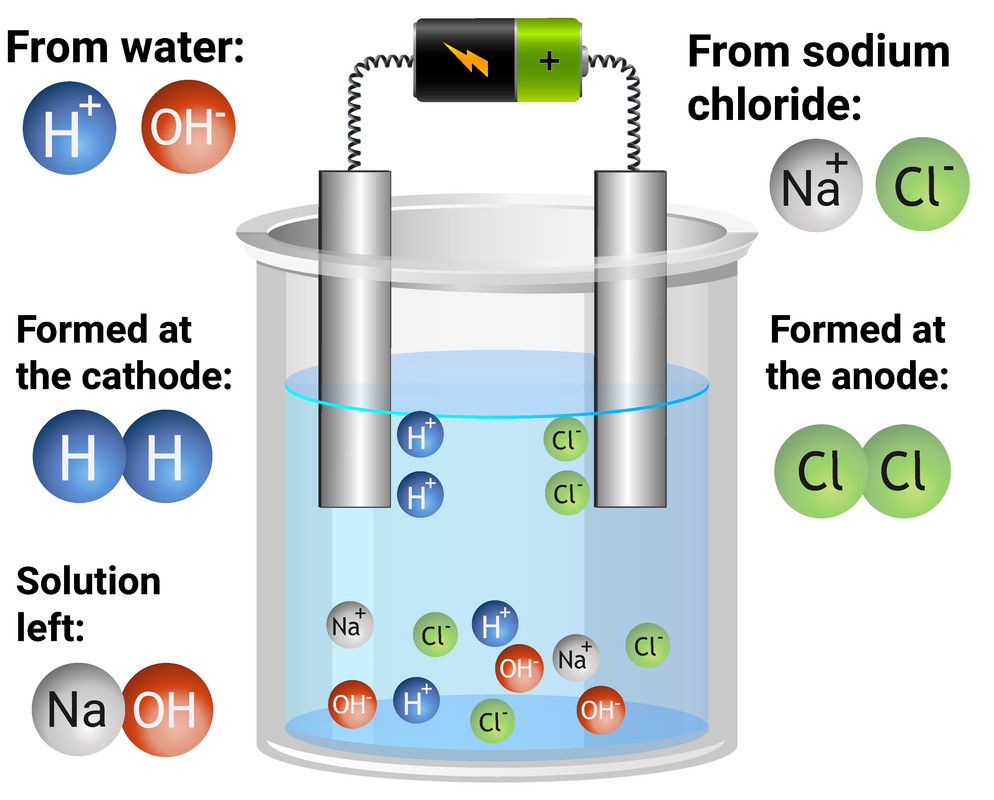
You need to be able to explain the formation of products during specific electrolysis experiments, when inert electrodes are used. In the table below, we can see which products are formed. If you are unsure why the following discharges happen, look again above at the rules of electrolysis.
| Electrolyte | At the anode (+) | At the cathode (-) |
|---|---|---|
| copper chloride (aq) (CuCl2) |
chlorine gas | copper metal |
| sodium chloride (aq) (NaCl) |
chlorine gas |
hydrogen gas |
| sodium sulfate (aq) (Na2SO4) |
oxygen gas | hydrogen gas |
| water acidified with sulfuric acid (H+/H2O) |
oxygen gas | hydrogen gas |
| molten lead bromide (PbBr2) |
bromine gas | lead metal |
Electrolysis of Copper
Purifying copper
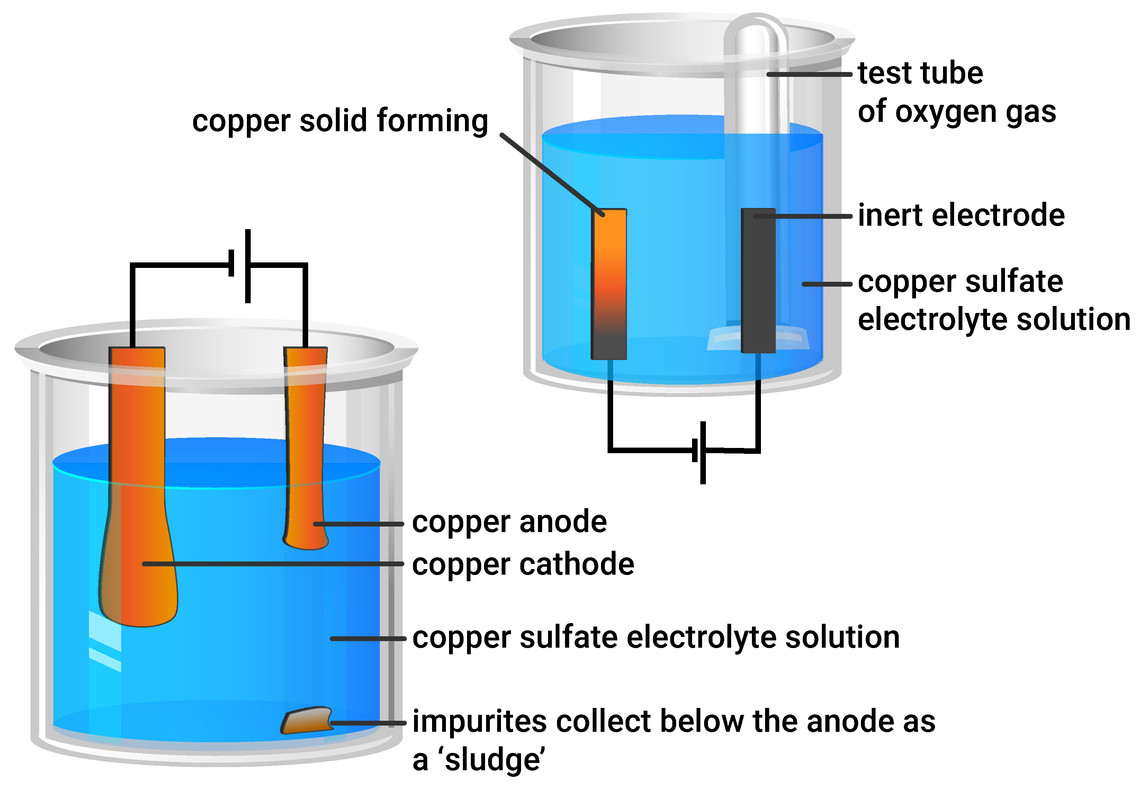
Copper can be purified by electrolysis. Electricity is passed through solutions containing copper compounds, such as copper sulfate. In this process, the anode would be made from impure copper and the cathode made from pure copper.
Copper ions are positive (Cu2+), so move to the negative electrode (cathode). During electrolysis, the anode loses mass as copper dissolves (as the ions are moving to the cathode), and the cathode gains mass as copper is deposited.
Impurities will form under the impure copper anode, in the form of a 'sludge'. This shouldn't be immediately thrown away, as it may contain valuable metals like silver.
Electrolysis of copper sulfate with graphite electrodes
Using inert electrodes (such as graphite electrodes) will still produce copper deposits at the cathode. However, rather than the anode mass decreasing (like it does in copper purification) we will produce oxygen gas. This can be tested for using a glowing splint, and if it relights - then oxygen is present.
Half Equations
Higher Tier
A half equation shows what happens at each electrode during electrolysis, in terms of the electron transfer. It shows what happens when ions gain electrons (reduction) or lose electrons (oxidation). In half equations:
- electrons are shown as e-
- the numbers of atoms of each element must be the same on both sides (like in a balanced equation)
- the total charge on each side needs to be the same
At the cathode
Positive ions (cations) will gather at the cathode, and gain electrons. You will either need to write a half equation for a metal, or hydrogen gaining electrons.
Group one metals, and hydrogen, only have a +1 charge, and so only need to gain one electron, but notice how because hydrogen gas is made of two hydrogen atoms, we must start with 2 hydrogen ions:
Na+(aq) + e- → Na(s)
K+(aq) + e- → K(s)
2H+(aq) + 2e- → H2(g)
Cu2+(aq) + 2e- → Cu(s)
At the anode
Negative ions (anions) will gather at the anode, and lose electrons. You will either need to write a half equation for a halide, oxygen, hydroxide or a metal.
2Cl-(aq) - 2e- → Cl2(g)
2O2-(aq) - 4e- → O2(g)
4OH-(aq) - 4e- → 2H2O(l) + O2(g)
Cu(s) - 2e- → Cu2+(aq)