Hydrocarbons
Separate Chemistry 2
Homologous Series: Alkanes
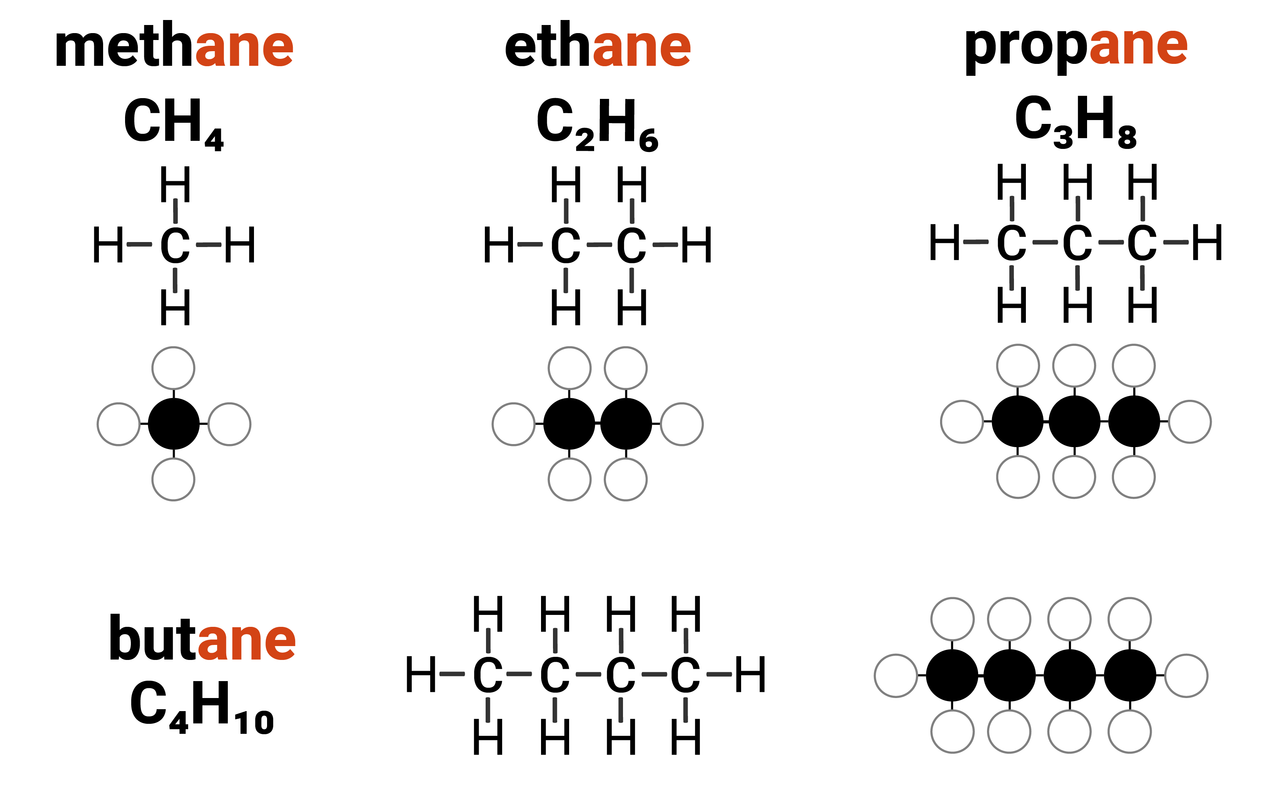
Alkanes are one example of a homologous series. They:
- have the general formula of CnH2n+2
-
this means that for every 1 carbon atom in an alkane, there are two times the amount of hydrogens... plus another two
- differ by CH2 in molecular formulae from neighbouring compounds
-
increasing the carbon chain length by 1 carbon atom, also increases the number of hydrogens by 2
- show a gradual variation in physical properties
-
boiling points increase with increased carbon chain length
viscosity increases with increased carbon chain length - have similar chemical properties
Because alkanes only contain single bonds between atoms, we describe them as saturated compounds. Think of when a sponge cannot hold any more water, it is "full". A saturated molecule can't fit any more single bonds into it. Alkanes can be considered "full".
Homologous Series: Alkenes
Alkenes are one example of a homologous series. They:
- have the general formula of CnH2n
-
this means that for every 1 carbon atom in an alkane, there are two times the amount of hydrogens
- differ by CH2 in molecular formulae from neighbouring compounds
-
increasing the carbon chain length by 1 carbon atom, also increases the number of hydrogens by 2
- show a gradual variation in physical properties
-
boiling points increase with increased carbon chain length
viscosity increases with increased carbon chain length
- have similar chemical properties
Because alkenes contain at least one double bond between carbon atoms, we describe them as unsaturated compounds. Think of when a sponge cannot hold any more water, it is "full". A saturated molecule can't fit any more single bonds into it. Alkenes can fit more single bonds in (once the double bonds are broken).
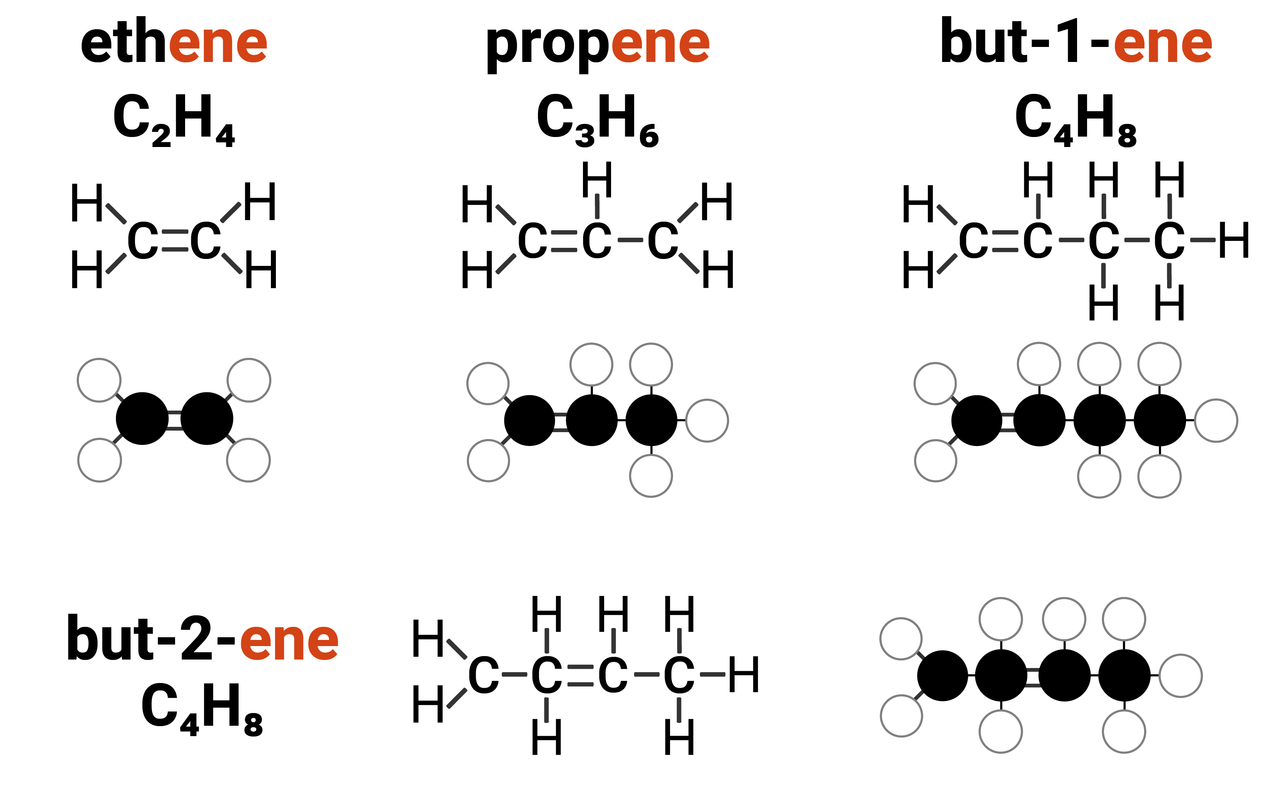
Alkenes can have more than one carbon-carbon double bond, however you will only ever be expected to draw an alkene with one of these bonds.
For ethene and propene, the double bond can only go in one place, however for any chains longer than 3 carbons - this can get a bit more complicated. You need to know the structures of all the isomers of butene that can form due to the double bond being in one of two places.
Reactions of Alkenes
Addition reactions
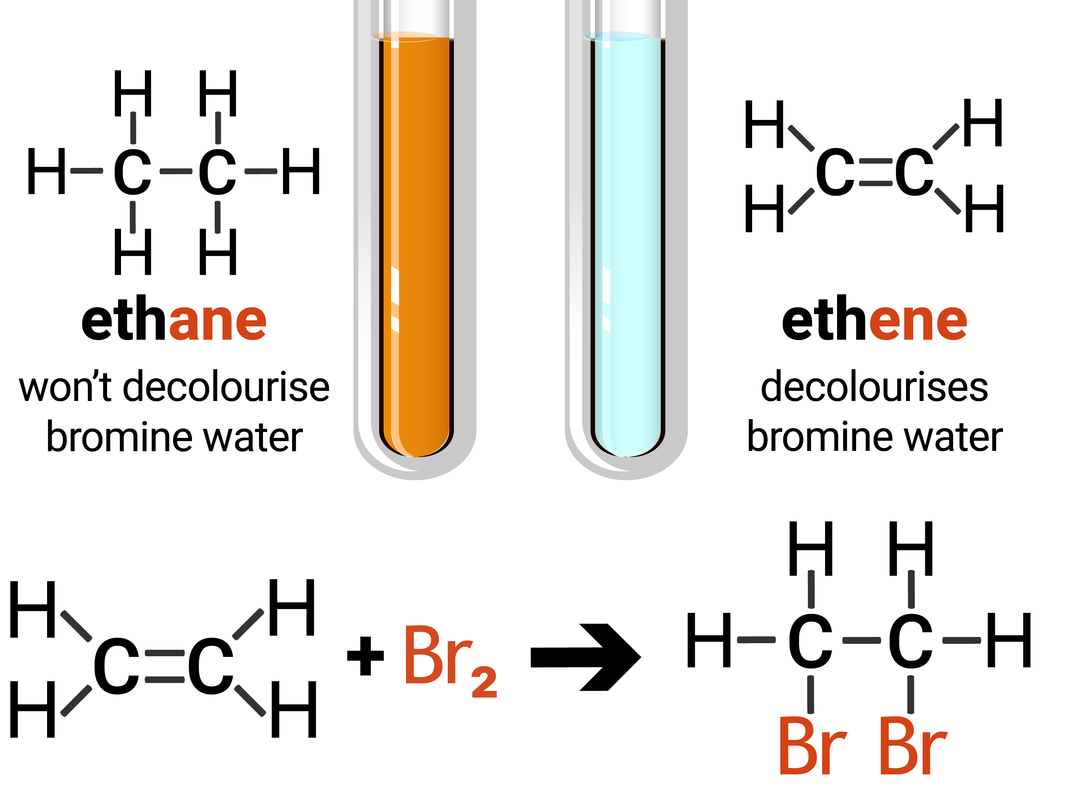
The functional group (double carbon bond, C=C) allows alkenes to undergo addition reactions. The reaction is an 'addition' reaction because one molecule combines with another molecule, forming one larger molecule and no other products.
The test for alkenes makes use of the addition reaction, and involves adding bromine water to a sample of hydrocarbon. If an alkane is present the solution stays orange-brown, but an alkene will turn the solution colourless. This is because the bromine is added across the carbon-carbon double bond.
Complete combustion
The complete combustion of alkanes and alkenes involves the oxidation of both the carbon and hydrogen atoms in hydrocarbons, producing carbon dioxide and water. For example:
ethane + oxygen → carbon dioxide + water
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(l)
ethene + oxygen → carbon dioxide + water
C2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(l)