
Polymers, Alcohols and Carboxylic Acids
Separate Chemistry 2
Polymers
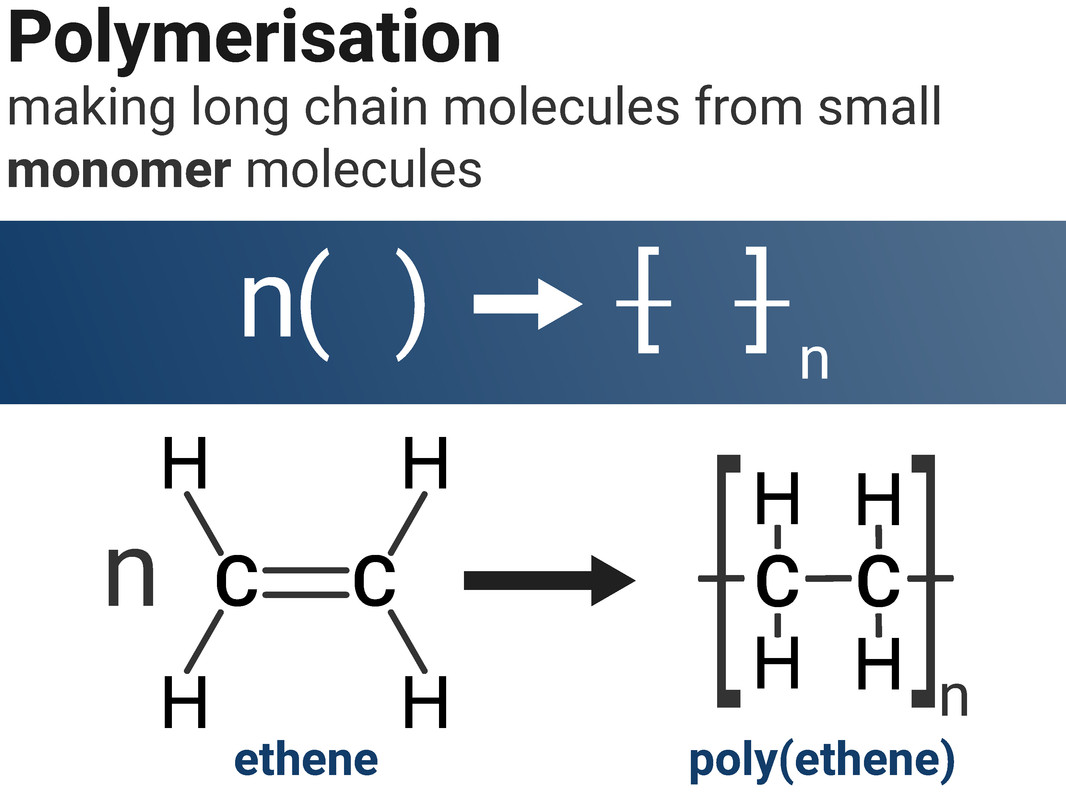
A polymer is a substance of high average relative formula mass, made up of small repeating units called monomers.
Addition Polymers
Ethene molecules can combine together in a polymerisation reaction to make poly(ethene). This type of reaction is called addition polymerisation, as we are adding lots of the same monomer together to make one product. The C=C double bond in ethene breaks open to allow ethene molecules to join together.
We don't waste our time drawing out a whole polymer chain, as it contains far too many atoms. Instead, we show the structure of the repeating unit.
When we draw an equation to show an addition polymerisation, it is important that we write n next to both the monomer and the polymer to show large numbers of each are made. We must also draw square brackets around the polymer structure.
Polymers and their Problems
| polymer | properties | uses |
|---|---|---|
| poly(ethene) |
flexible, can be made into thin film | carrier bags, bottles, food wrap |
| poly(propene) |
flexible, strong, shatter-resistant | buckets, bowls, ropes, carpet |
| poly(chloroethene), PVC |
tough, insulator, either hard or flexible | electrical insulation, windows, pipes |
| poly(tetrafluoroethene), PTFE, Teflon™ | slippery, unreactive | non-stick coatings for pans |
There are many problems associated with polymers including the:
- availability of starting materials
- persistence in landfill sites, due to non-biodegradability (microorganisms are unable to break them down)
- greenhouse gases and toxic gases produced during disposal by combustion (to power homes)
- requirement to sort polymers so that they can be melted and reformed into a new product

Recycling polymers is great as it reduces the problems of disposal, and reduces our use of crude oil - but it is difficult and expensive to sort each of the polymers to be recycled.
Biological Polymers
Biological polymers are made naturally by living organisms.
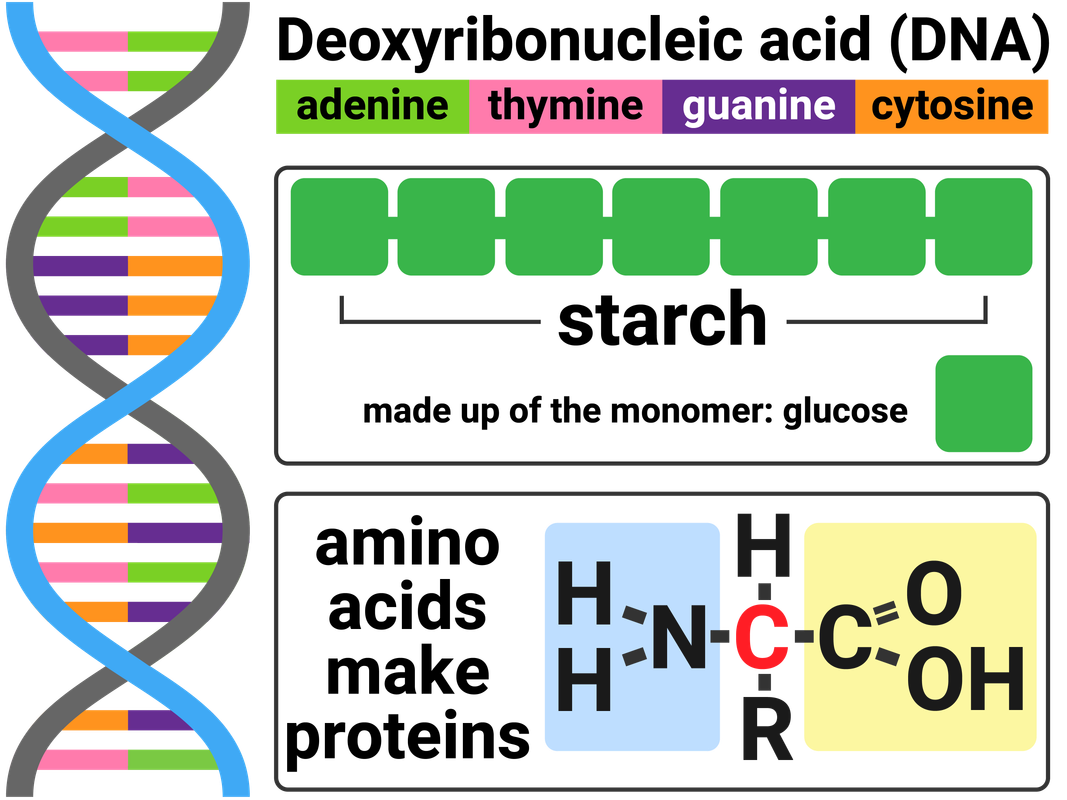
DNA is a polymer made from four different monomers called nucleotides. Most DNA molecules are made from two polymer chains. These polymer chains join together in the shape of a double helix. Each nucleotide is made of a sugar molecule, a base (A, C, T or G), and a phosphate group.
Carbohydrates include substances such as sugar, starch, and cellulose. These molecules are made from carbon, hydrogen and oxygen. Simple sugars, like glucose, are called monosaccharides. Glucose monomers can join together in long chains to make a polysaccharide called starch.
Proteins are another example of polymers. There are 21 different amino acids that can be arranged to make proteins we need to survive. Many proteins are enzymes, and/or are essential to our biological functions.
Condensation Polymers
Higher Tier
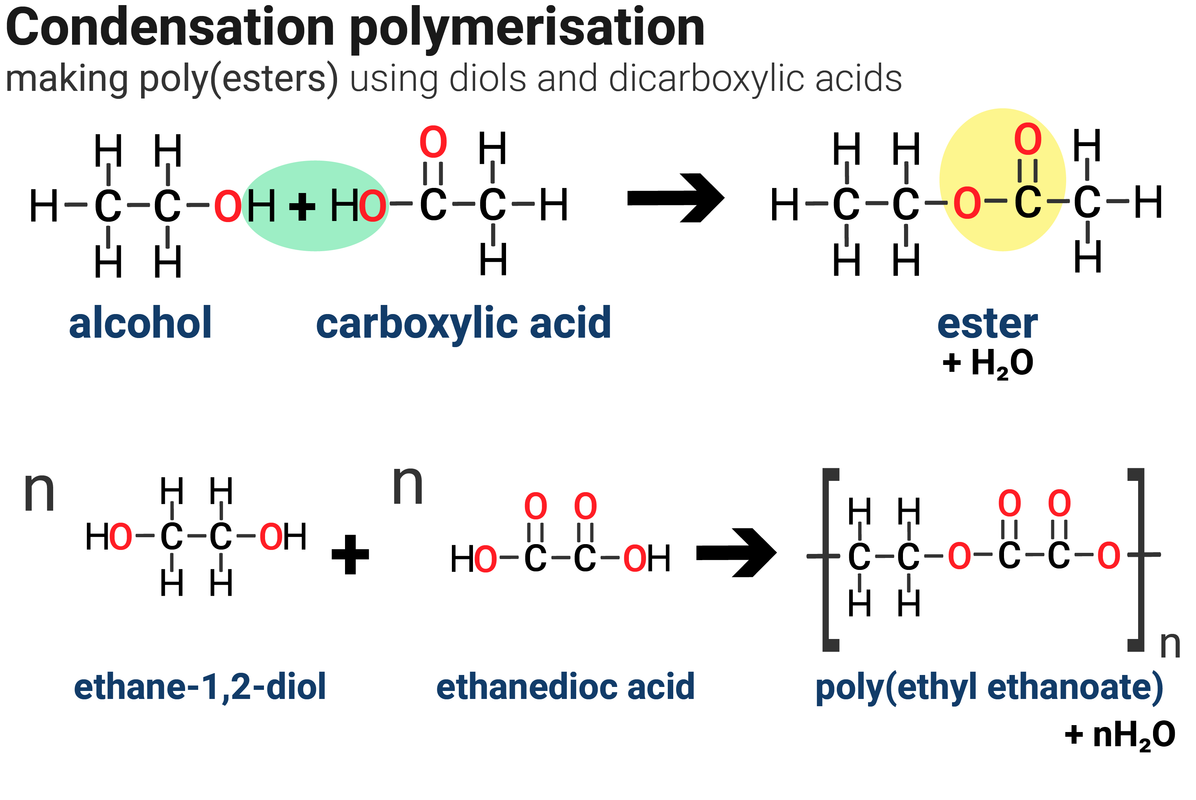
An ester forms when an alcohol reacts with a carboxylic acid. For example:
methanol + ethanoic acid → methyl ethanoate + water
Methyl ethanoate is an ester. For every one ester molecule made, there is a water molecule formed.
Condensation polymerisation is another way of making polymers. Instead of forming just the polymer molecule as the only product, two products form:
- a polymer molecule
- a small molecule, normally water
A polyester requires two different monomers to form a polymer. There must be one of each of the following different monomers:
- a 'dicarboxylic acid' - monomer contains two carboxylic acid groups, –COOH
- a 'diol' - monomer contains two alcohol groups, –OH
Note that:
- both ends of each monomer molecule have a functional group, so can react with another monomer molecule
- one molecule of water is formed every time an ester link is formed
Homologous Series: Alcohols
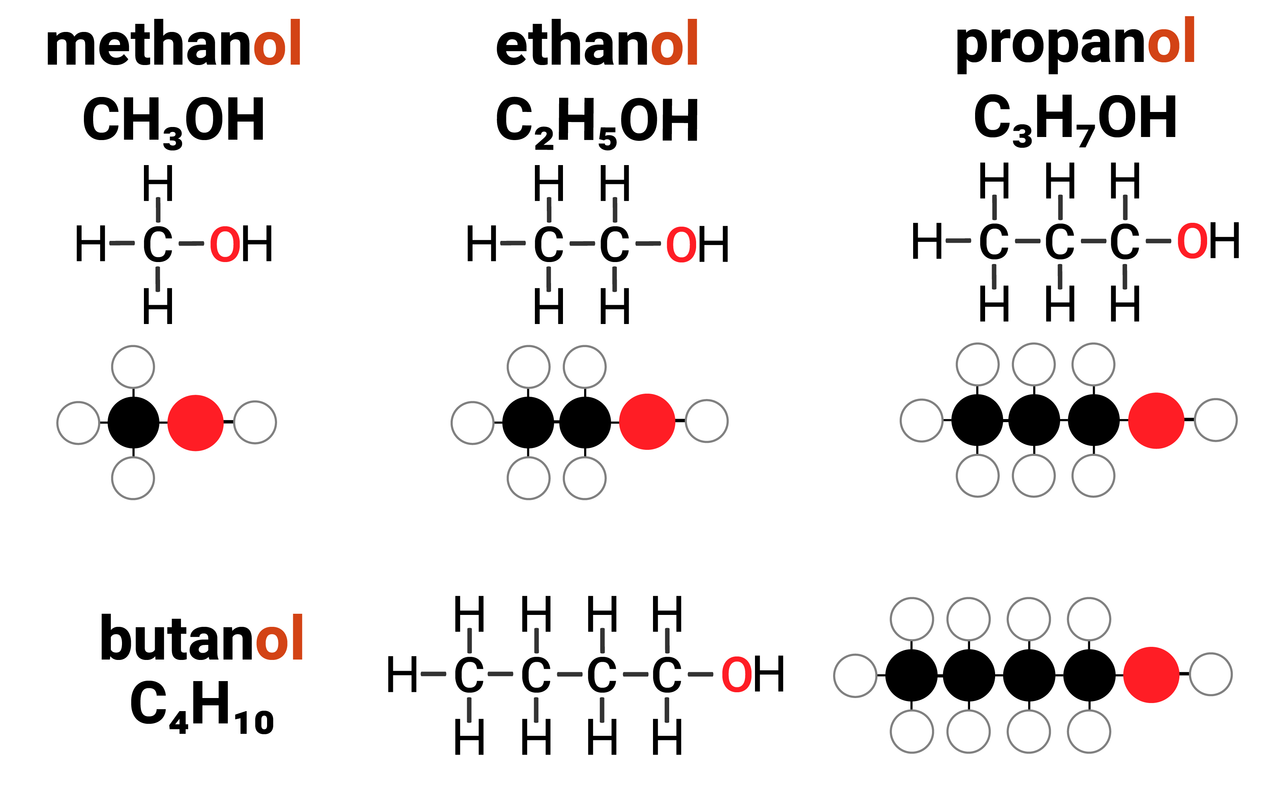
Alcohols are one example of a homologous series. They:
- have the general formula of CnH2n+1OH
-
this means that for every 1 carbon atom in an alcohol, there are two times the amount of hydrogens... plus one more, and an OH group
- differ by CH2 in molecular formulae from neighbouring compounds
-
increasing the carbon chain length by 1 carbon atom, also increases the number of hydrogens by 2
- show a gradual variation in physical properties
-
boiling points increase with increased carbon chain length
viscosity increases with increased carbon chain length
- have similar chemical properties
Alcohols have the functional group of -OH (hydroxyl) which gives rise to the ending of alcohol. It is responsible for the general reactions that all alcohols can do.
The longer the carbon chain of an alcohol, the more energy the molecule stores. When burned, this energy is released in the form of heat and can be measured using a calorimeter.
Production of Ethanol
Fermentation
glucose → ethanol + carbon dioxide
Most ethanol is made from plants, making it a renewable resource. The process is called fermentation, and requires:
- water
- yeast enzymes
- an absence of oxygen (an anaerobic process)
- temperatures between 25 - 35 °C
The yeast dies when the ethanol concentration reaches about 15%.
Fermentation is a slow reaction and takes several days or weeks to finish. If any air is present, the oxygen oxidises the ethanol to make ethanoic acid, making the drink taste of vinegar.
Purification
Fermentation will produce a dilute solution of ethanol, and needs to be purified to remove water and other liquids (removed by fractional distillation), insoluble substances (removed by filtration), to produce pure ethanol.
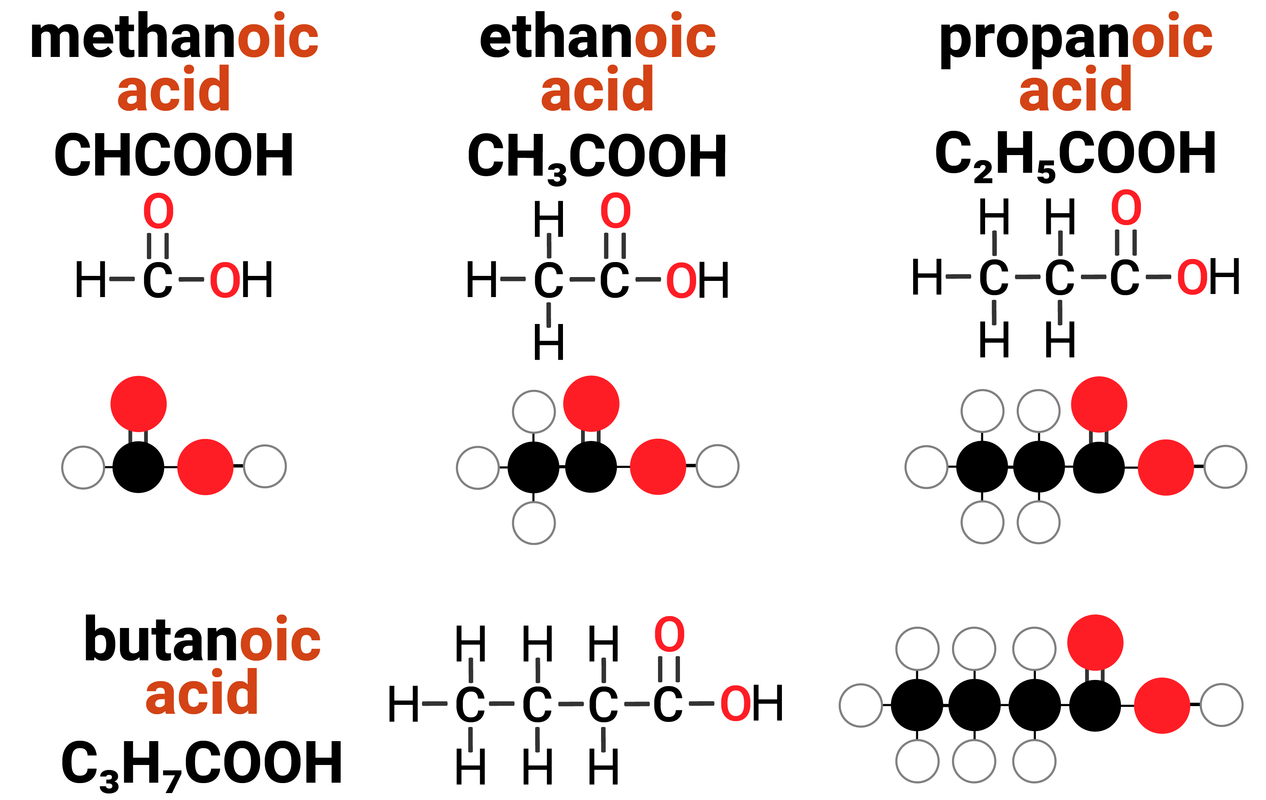
Homologous Series: Carboxylic Acids
Carboxylic acids are one example of a homologous series. They:
- have the general formula of CnH2n+1COOH
-
this means that for every 1 carbon atom in a carboxylic acid, there are two times the amount of hydrogens... plus one more, and a COOH group
- differ by CH2 in molecular formulae from neighbouring compounds
-
increasing the carbon chain length by 1 carbon atom, also increases the number of hydrogens by 2
- show a gradual variation in physical properties
-
boiling points increase with increased carbon chain length
viscosity increases with increased carbon chain length
- have similar chemical properties
Carboxylic acids have the functional group of -COOH (carboxyl) which gives rise to the ending of carboxylic acid. It is responsible for the general reactions that all carboxylic acids can do.
Reactions of Alcohols and Carboxylic Acids
Alcohols
- colourless liquids that dissolve in water to form neutral solutions
- can be dehydrated to form alkenes (produces a water molecule)
- will react with the alkali metals to produce hydrogen and a metal hydroxide (like sodium would with water)
- burn in air to produce carbon dioxide and water (complete combustion)
- can be oxidised to make a carboxylic acid in the presence of an oxidising agent (shown as [O])
Carboxylic acids
- dissolve in water to form acidic solutions (pH values less than 7)
- react with metals to form a salt and hydrogen
- react with bases to form a salt and water
- react with carbonates to form a salt, water and carbon dioxide