Introducing the Practical
Apparatus set up to measure the increase in temperature when the different fuels given are burnt.
This must lead to a comparison of the results for the four alcohols.
Risk Asessment
As a general rule, eye protection (goggles) must be worn for all practicals.
| hazard | possible harm | precaution |
|---|---|---|
| alcohols (highly flammable) |
burns to the skin, fire, and damage by fire |
light wick with a lit splint, don't move a spirit burner while alight, keep lab well ventilated |
| methanol (toxic) |
danage to health when in contact with the skin |
avoid skin contact, wear gloves |
This risk assessment is provided as an example only, and you must perform your own risk assessment before doing this experiment.
Apparatus
Each group will need:
alcohol burners
150 ml conical flasks
thermometers
retort stands and clamps
access to a balance (at least 2 d.p.)
100 ml measuring cylinders
lighters or matches
methanol
ethanol
propan‐1‐ol
butan‐1‐ol
Experiment Set-up
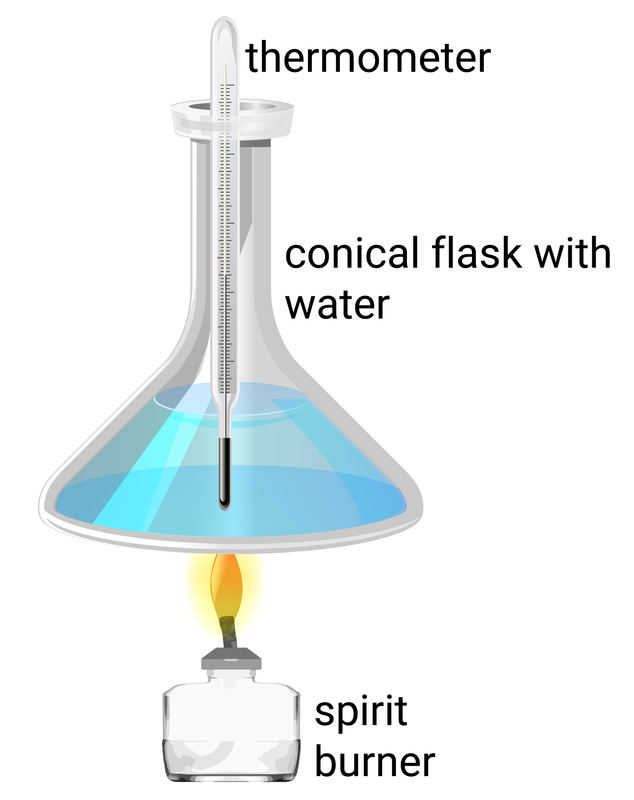
Method
- secure a conical flask over a spirit burner so that the lid of the burner can be removed and replaced safely
- measure and record the mass of a spirit burner with its lid
- use a measuring cylinder to add 100 ml of cold water to the flask, and then record its temperature
- place the spirit burner underneath the flask, remove the lid and light the wick
- stir the water carefully with the thermometer until the temperature has increased by 20°C, then replace the lid to put the flame out
- measure and record the new mass of the spirit burner (with lid), and the maximum temperature that the water reached
- repeat steps 2-6 with different alcohols, starting with new water every time
Results and Analysis
| temperature of water at start (°C) |
temperature of water at end (°C) |
change in temperature (°C) |
mass of spirit burner at start (g) | mass of spirit burner at end (g) | change in mass (g) | |
|---|---|---|---|---|---|---|
| methanol | 20 | 40 | 235.60 | 235.05 | ||
| ethanol | ||||||
| propanol | ||||||
| butanol |
The change in mass is equal to the mass of fuel burned.
For each experiment, calculate the change in temperature and the change in mass.
How is the temperature rise of water related to the number of carbon atoms in the alcohol molecules?
Exam Question and Model Answer
An investigation was carried out to find out which alcohol gives out the most energy when burned. The apparatus used included (see diagram above):
alcohol burners
150 ml conical flask
thermometer
retort stands and clamp
balance
100 ml measuring cylinder
Describe and explain three improvements that could be made to the method to obtain experimental values closer to the theoretical values of energy released.
[4 marks]
Level 1 (1-2 marks)
Wrapping the equipment in insulation or at least putting a lid on the beaker;
would reduce the heat lost after it is transfered to the water.
Level 2 (3-4 marks)
They could use a better conductor, so use a copper can instead of glass beaker - this would allow more heat to be transferred to the water.
Wrapping the equipment in insulation or at least putting a lid on the beaker would reduce the heat lost after it is transfered to the water.
Level 3 (5-6 marks)
The student should reduce the heat loss between the flame and the beaker by enclosing the apparatus/surrounding the equipment to recuce any drafts.
They could also use a better conductor, so use a copper can instead of glass beaker - this would allow more heat to be transferred to the water.
Wrapping the equipment in insulation or at least putting a lid on the beaker would reduce the heat lost after it is transfered to the water.








