Apparatus and techniques that the practical must use or cover
Safe use of a range of equipment to purify and/or separate chemical mixtures including evaporation, filtration, crystallisation, chromatography and distillation.
Safe use of appropriate heating devices and techniques including use of a Bunsen burner and a water bath or electric heater.
Use of appropriate apparatus to make and record a range of measurements accurately, including mass, time, temperature, and volume of liquids and gases.
Risk Asessment
As a general rule, eye protection (goggles) must be worn for all practicals.
| hazard | possible harm | precaution |
|---|---|---|
| hot apparatus |
skin burns |
allow apparatus to cool before handling it |
| Bunsen Burner |
skin burns, fire | keep hair and clothes tucked in, and do not bring flammable solvents near to the flame |
| harmful solvents |
skin irritation, breathing problems | avoid skin contact (wear gloves), and ensure adequate ventilation/use a fume cupboard |
This risk assessment is provided as an example only, and you must perform your own risk assessment before doing this experiment.
Apparatus
Each group will need:
a conical flask with a two‐hole bung with a thermometer and delivery tube
test tube
beaker (250 or 400 ml)
crushed ice
Bunsen burner
tripod
gauze
heat‐resistant mat
ink
antibumping granules
250 ml beaker
chromatography paper
a splint or pencil or glass rod/paper clips
selection of different coloured marker pens or felt‐tip pens
solvent (water)
Experiment Set-up
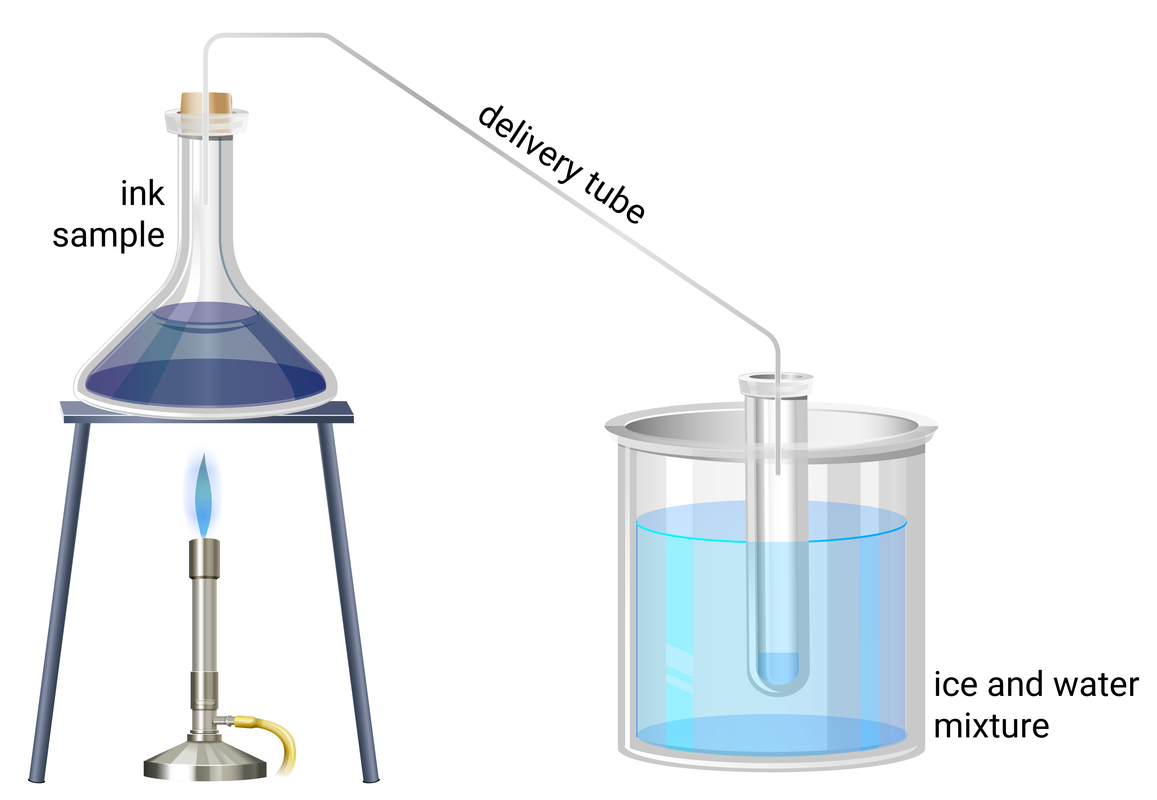
Simple Distillation
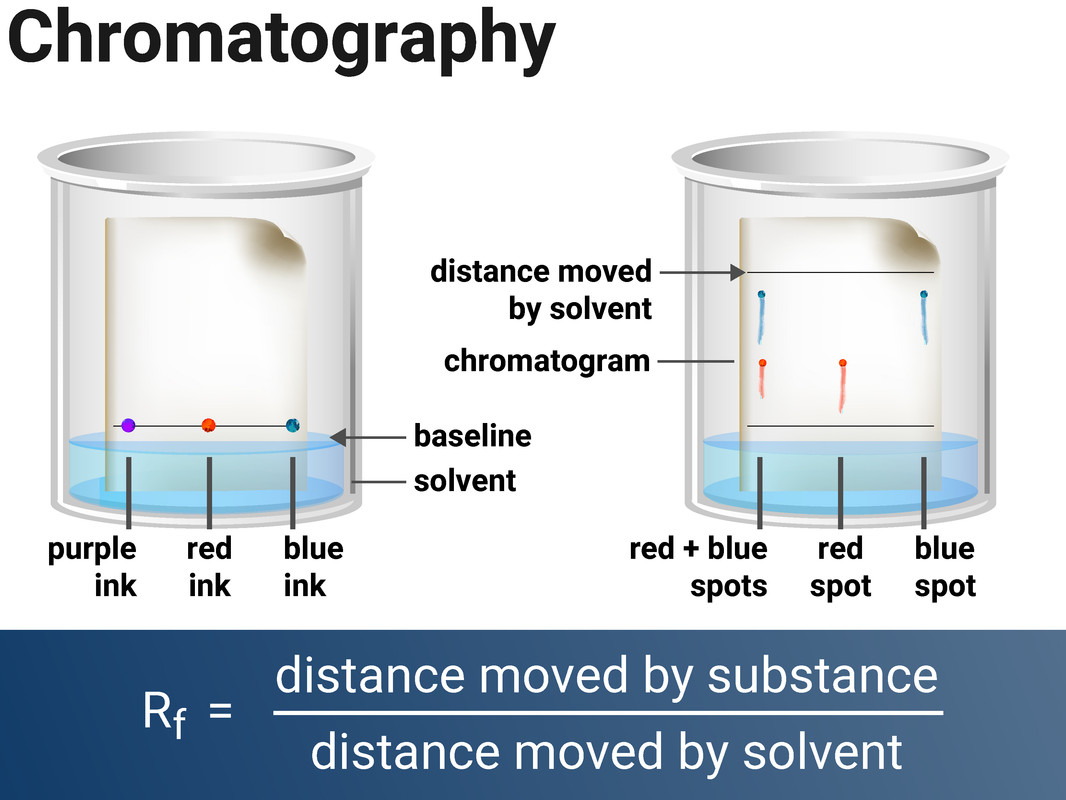
Paper Chromatography
Method
Simple Distillation
- add about 20 ml of ink to a conical flask
- connect the flask to a delivery tube and set up the apparatus as shown above
- heat the flask using a Bunsen burner until boiling, then reduce the heat for a gentle boil
- collect a small sample of the distilled solvent, then turn the Bunsen Burner off
Paper Chromatography
- draw a pencil line across chromatography paper (about 1 cm above the bottom)
- use a pipette, or capillary tube, to add small spots of each ink to the line on the paper (equally spread across the paper)
- place the paper into a container with a suitable solvent (e.g. water) in the bottom - making sure the pencil line is above the water line
- allow the solvent to move through the paper, but remove the chromatogram before it reaches the top
- allow the chromatogram to dry, then measure the distance travelled by each spot and by the solvent
Results and Analysis
Simple Distillation
What is the appearance of the distilled solvent?
Explain any difference in the appearance of the solvent and ink.
At what temperature did the thermometer read as your solvent condensed?
How does this compare with the boiling points of common solvents like water, ethanol or propanol?
Paper Chromatography
| ink | spot colour | distance travelled by spot (mm) |
|---|---|---|
Calculate the Rf value of each spot.
Compare the Rf values/colours of each spots in the different inks - what are the similarities and differences?
Exam Question and Model Answer
A new type of waterproof pen has just been released, and it contains ink that is insoluble in water. A company suspects it may be a copy of their pen's ink. Describe how they should use paper chromatography to identify whether the inks are the same.
[6 marks]
Level 1 (1-2 marks)
Place a spot of the inks on the paper, and place the paper in a beaker with water (not covering the pencil line)
Hang the piece of paper in a beaker and let the water rise through the paper
Level 2 (3-4 marks)
Place a spot of the new pen's ink from the pen on chromatogaphy paper, and place a spot of the original ink on there too.
Hang the piece of paper in a beaker, and add a small amount of solvent (not water, e.g. ethanol) as to not cover the pencil line.
Allow the solvent to rise up the paper.
Level 3 (5-6 marks)
Draw a line (in pencil) across a piece of chromatography paper, about 1 cm above the bottom.
Place a spot of the new pen's ink from the pen on the pencil line, and place a spot of the original ink on the line.
Hang the piece of paper in a beaker, and add a small amount of solvent (not water, e.g. ethanol) as to not cover the pencil line.
Allow the solvent to rise up the paper.
Measure how far the solvent moved, as well as each spot on the chromatogram, and calculate the Rf values of each spot.
If the Rf values match, then the ink is a copy.








