Introducing the Practical
A titration is carried out to determine the volume of hydrochloric acid required to neutralise a solution of unknown concentration of sodium hydroxide.
An indicator must be used to determine the end point.
Standard procedure for a titration must be carried out, such as the use of a white tile and swirling the conical flask to obtain an accurate end point.
The data must then be used to determine the concentration of the unknown solution.
Risk Asessment
As a general rule, eye protection (goggles) must be worn for all practicals.
| hazard | possible harm | precaution |
|---|---|---|
| dilute sodium hydroxide solution |
skin irritation and serious eye irritation |
wear gloves, use a pipette filler |
| dilute hydrochloric acid |
skin irritation and eye irritation |
fill burette slowly (below eye level), using a funnel |
This risk assessment is provided as an example only, and you must perform your own risk assessment before doing this experiment.
Apparatus
Each group will need:
burette
burette stand or retort stand with burette clamp
plastic funnel to fit in top of burette
25.0 ml pipette
pipette filler
conical flask
white tile
phenolphthalein indicator
hydrochloric acid
sodium hydroxide solution
access to distilled/de‐ionised water
Experiment Set-up
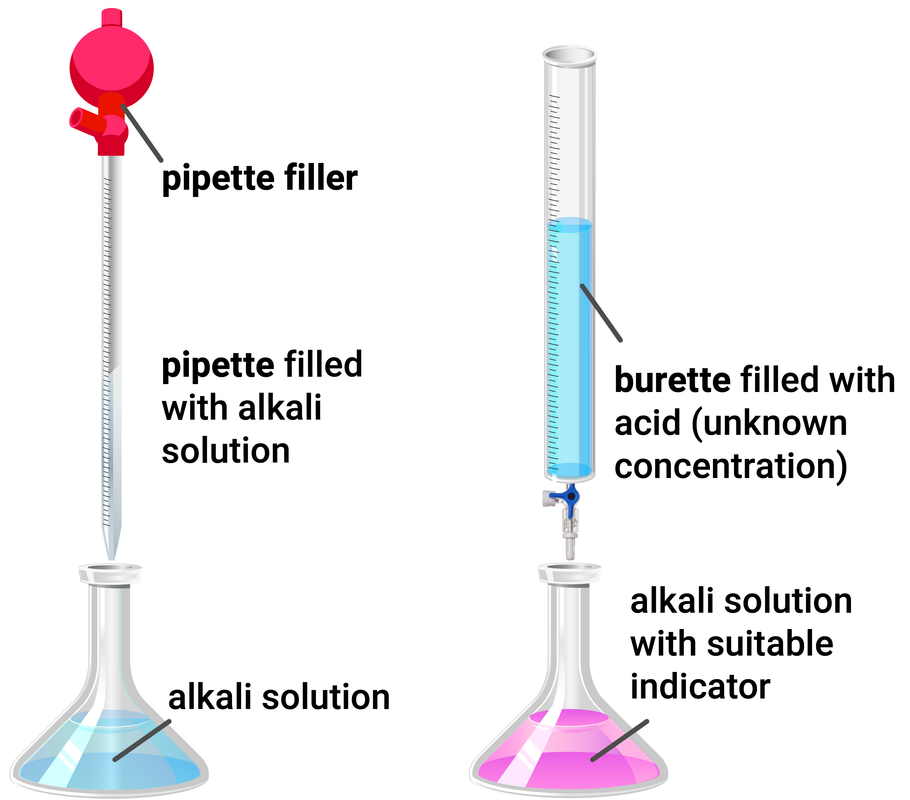
Method
- use a pipette and pipette filler to add 25 ml of sodium hydroxide to a clean conical flask
- add a few drops of phenolphthalein indicator and place the conical flask on a white tile
- fill the burette with hydrochloric acid and record the starting volume
- slowly open the tap of the burette, and add the acid to the conical flask, swirling to mix
- stop adding the acid when the end-point is reached (when the colour permanently changes from pink to colourless) and record your final volume
- repeat steps 1-5 until you get concordant titres (results are within 0.10 ml of each other)
Results and Analysis
| rough | run 1 | run 2 | run 3 | |
|---|---|---|---|---|
| final volume (ml) | 26. 55 | 26.35 | 26.80 | 26.25 |
| starting volume (ml) | 0.00 | 0.15 | 0.50 | 0.00 |
| titre (ml) | 26.55 | 26.20 | 26.30 | 26.25 |
Readings should be recorded to two decimal places, ending in 0 or 5.
The titre is the volume added (the difference between the final and starting volumes).
Select at least two concordant titres (these are titres within 0.10 ml of each other) and work out an average titre.
Use this volume of acid to work out its concentration.
Exam Question and Model Answer
A student has to check if two samples of hydrochloric acid, A and B, are the same concentration.
Describe how the student could use the apparatus (burette, pipette, conical flask, white tile) and the solutions (indicator, HCl A, HCl B, NaOH solution) to carry out titrations.
[6 marks]
Level 1 (1-2 marks)
Use a pipette to measure the acid into a conical flask, then add alkali from the burette.
Level 2 (3-4 marks)
Add hydrochloric acid, using a pipette, into a conical flask.
Fill a burette with the sodium hydroxide solution, then add a few drops of indicator into the conical flask.
Slowly add the alkali to the flask and swirl the flask whilst looking for a colour change.
Once a sudden colour change has occurred, stop adding alkali, and record the volume of alkali added from the burette.
Level 3 (5-6 marks)
Ensure you are wearing safety goggles and measure 25 ml of hydrochloric acid A, using a pipette and pipette filler, into a conical flask.
Fill a burette safely with the sodium hydroxide solution, then add a few drops of indicator into the conical flask.
Slowly add the alkali, drop by drop, to the conical flask and swirl the flask whilst looking for a colour change. Using a white tile under the flask can help to see this.
Once a sudden colour change has occurred, stop adding alkali, and record the volume of alkali added from the burette.
Repeat this again for the hydrochloric acid B - and if the same volume of alkali neutralises the acids, the two acids are of the same concentration.








