Aims of Experiment
To prepare a pure, dry sample of a soluble salt from an insoluble oxide or carbonate.
In this experiment you will:
- react sulfuric acid with insoluble copper (II) oxide to prepare an aqueous solution of the salt copper sulfate
- separate out unreacted copper (II) oxide by filtration
- prepare pure, dry crystals of copper sulfate from the solution
Risk Asessment
As a general rule, eye protection (goggles) must be worn for all practicals.
| hazard | possible harm | precaution |
|---|---|---|
| sulfuric acid |
concentrated acid is corrosive and damages skin and clothes |
use dilute sulfuric acid (only an irritant, wash hands if spillage) |
| Bunsen Burner/hot apparatus |
burns, hair or clothing catching fire |
do not touch the apparatus, tie hair/tuck in loose clothes |
| boiling water bath |
skin burns | ensure the boiling water bath is stable, and you are standing up |
| hot salt solution spitting |
damage to eyes and skin | avoid standing over the hot apparatus |
This risk assessment is provided as an example only, and you must perform your own risk assessment before doing this experiment.
Apparatus
Each group will need:
evaporating basin
spatula
stirring rod
filter funnel
filter paper
tongs
sulfuric acid
copper(II) oxide
250 ml conical flask
100 ml beaker
Bunsen burner
gauze
tripod stand
heat-resistant mat
watch glass
100 ml measuring cylinder
Experiment Set-up
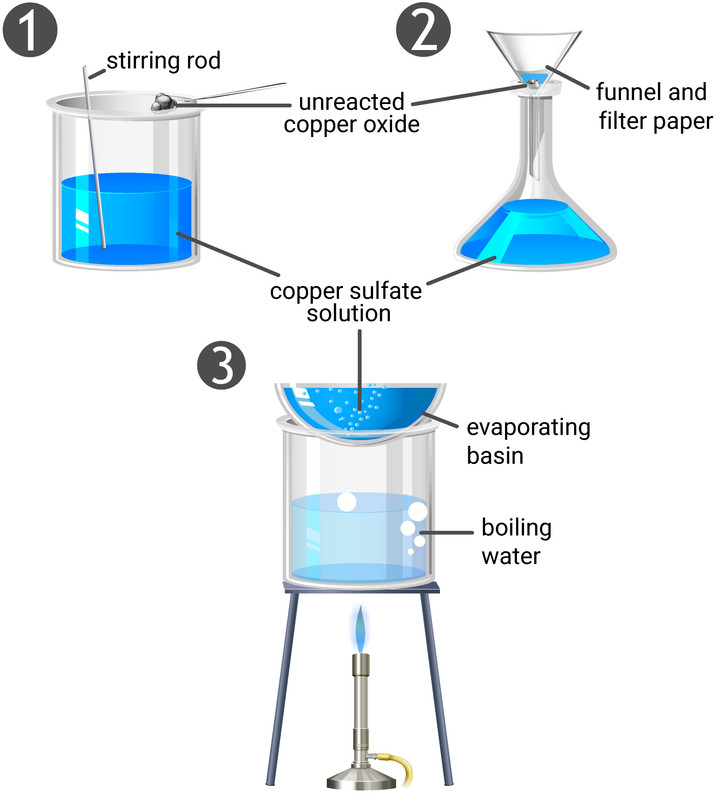
Method
- use a measuring cylinder to add 40 ml of sulfuric acid in a beaker
- gently heat the beaker in a water bath for a couple of minutes
- carefully add a spatula of copper oxide powder to the beaker and stir the solution with a glass rod,
- keeping adding more copper oxide powder until it no longer disappears (add in excess)
- filter the mixture to remove the excess copper oxide, then pour the filtrate (the copper sulfate solution) into an evaporating basin
- place the evaporating basin above a water bath, and heat the copper sulfate solution to evaporate off half of the water
- pour the solution into a watch glass and leave on the side to allow all of the water to evaporate
Results and Analysis
Why was it necessary to warm the sulfuric acid?
How did you know when the copper oxide was present in excess?
Why is a water bath used to evaporate the water from the copper sulfate solution instead of heating the evaporating basin directly with a Bunsen burner?
Why should you not evaporate all of the water from the copper sulfate solution?
Exam Question and Model Answer
This question is about making copper salts. Outline a safe plan the student could use to make pure, dry, crystals of the soluble salt copper sulfate from an insoluble metal oxide and dilute acid.
(Apparatus available: stirring rod, spatula, beaker, filter paper and funnel, evaporating basin, Bunsen burner, tripod, gauze and mat, and conical flask)
[6 marks]
Level 1 (1-2 marks)
Add the metal oxide to the dilute acid. Stir them.
Filter the solution and then evaporate off the water.
Level 2 (3-4 marks)
Safely measure 25 ml sulfuric acid into a conical flask.
Add copper oxide to the flask, and then heat the acid until no more copper oxide will react.
Pour the contents of the conical flask into an evaporating basin.
Filter the solution.
Heat this gently and stop heating once crystals start to form.
Leave the solution to evaporate overnight.
Level 3 (5-6 marks)
Ensure you are wearing safety goggles and measure 25 ml sulfuric acid into a conical flask. Sulfuric acid is corrosive.
Add excess copper oxide to the flask, and then heat the acid gently using the Bunsen burner, whilst stirring the solution, until no more copper oxide will react.
Allow the solution to cool, then any remaining copper oxide must be removed using a funnel/filter paper, by filtration.
Pour the contents of the conical flask into an evaporating basin. Heat this gently on a tripod and gauze, on top of a beaker half-filled with water. Stop heating once crystals start to form.
Leave the solution to evaporate overnight, then remove the crystals and dry them.








