Aims of Experiment
Investigate what happens when two different aqueous solutions are electrolysed using inert electrodes.
In this experiment you will:
- use a low voltage power supply and carbon rod electrodes to pass a current through two different salt solutions
- identify the element formed at the positive and negative electrodes for each solution
- add extra detail to a basic electrochemical diagram (provided)
Risk Asessment
As a general rule, eye protection (goggles) must be worn for all practicals.
| hazard | possible harm | precaution |
|---|---|---|
| copper sulfate solution |
skin and serious eye irritation |
wear gloves |
| DC electric supply |
electric shock |
switch off apparatus before touching, ensure electrodes do not ever touch |
| chlorine gas |
toxic, harm to health |
switch off apparatus after a few minutes, do not stand directly over the reaction, make sure the room is well ventilated |
This risk assessment is provided as an example only, and you must perform your own risk assessment before doing this experiment.
Apparatus
Each group will need:
low voltage supply (0‐12 V)
connecting leads
crocodile clips
two small test tubes (gas collecting)
matches/splints
blue litmus paper
100 ml beaker
stop watch
2 graphite rods
copper chloride solution
sodium chloride solution
Experiment Set-up
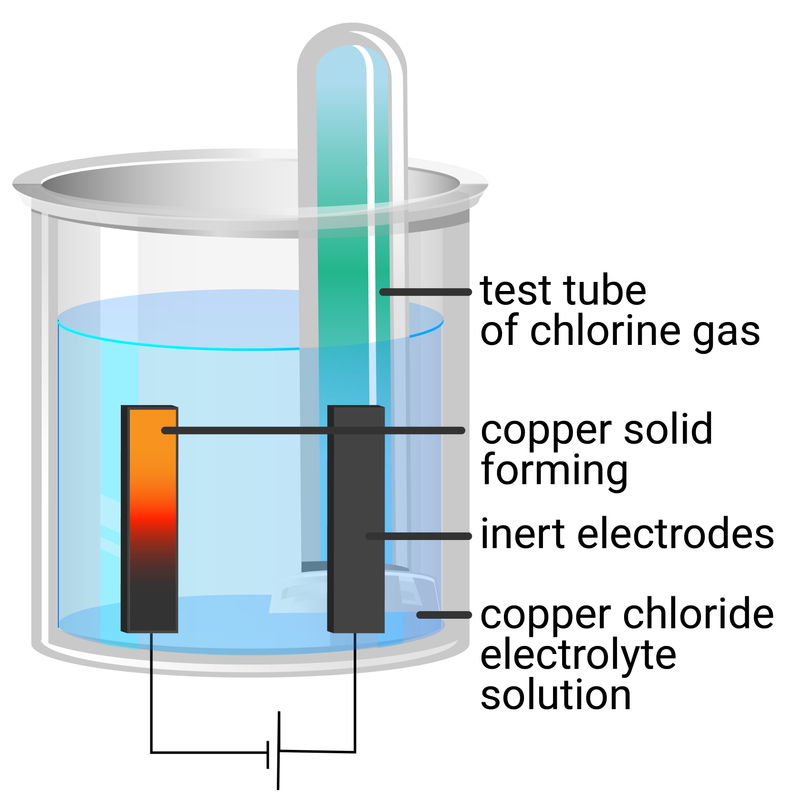
copper chloride solution
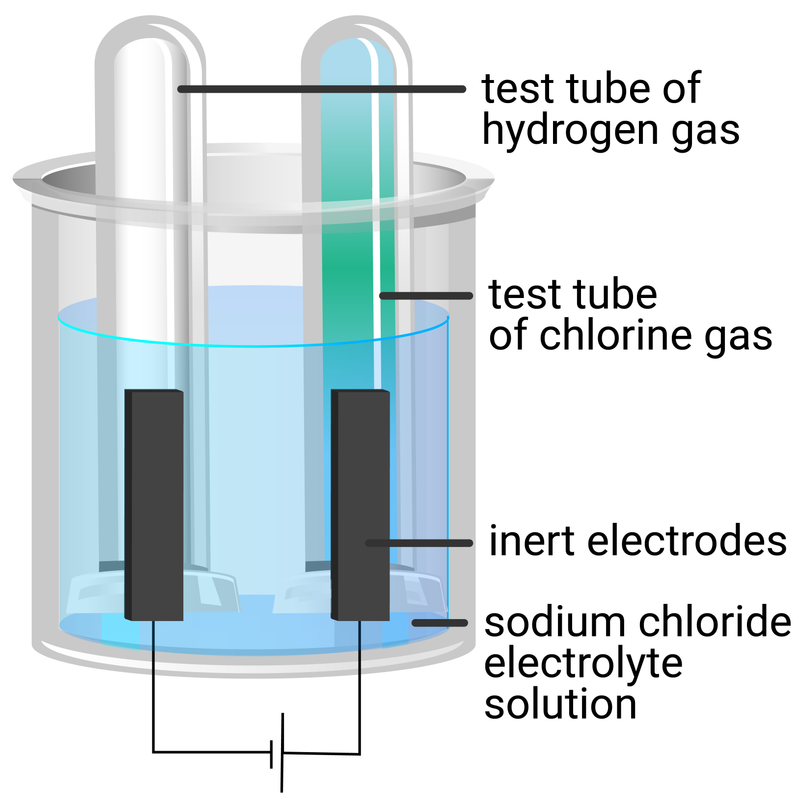
sodium chloride solution
Method
- use a measuring cylinder to add 40 ml of copper chloride solution into a beaker
- place two graphite rods into the copper sulfate solution - attaching one electrode to the negative terminal of a dc supply, and the other electrode to the positive terminal
- place two small test tubes over each electrode to collect any gases produced
- turn on the power supply and observe what happens at each electrode
- test any gas produced by holding a piece of blue litmus next to the electrode, or by holding a lit splint next to it
- record your observations and the results of your tests
- rinse the electrochemical cell apparatus and collect a new set of electrodes
- repeat steps 1‒6 using sodium chloride solution
Results and Analysis
| solution used | negative electrode (cathode) | positive electrode (anode) | ||
|---|---|---|---|---|
| observation | element produced | observation | element produced | |
| copper(II) chloride | brown solid forms | copper metal | pale green gas produced | chlorine gas |
| sodium chloride | colourless gas produced | hydrogen gas (squeeky pop test) | pale green gas produced | chlorine gas (blue litmus test) |
Descibe the tests for both hydrogen and chlorine.
Explain why copper was deposited at the cathode for copper(II) chloride, but hydrogen gas was produced for sodium chloride.
Higher Tier - Write half equations for each of the electrodes in both experiments.
Exam Question and Model Answer
A student conducts an investigation to find out what is produced during the electrolysis of sodium sulfate.
Describe how the student could carry out an investigation, and show (using ions) what is given off at each electrode.
[6 marks]
Level 1 (1-2 marks)
Add sodium sulfate solution to a beaker, and connect two electrodes to a power supply.
Completely fill two small test tubes with sodium sulfate solution and position a test tube over each electrode, then turn on the power supply and observe what happens at each electrode.
hydrogen gas will form at the negative electrode
oxygen gas will form at the positive electrode
Level 2 (3-4 marks)
Wearing safety glasses, add sodium sulfate solution to a beaker, and connect two inert electrodes (make sure they do not touch) to a power supply.
Completely fill two small test tubes with sodium sulfate solution and position a test tube over each electrode, then turn on the power supply and observe what happens at each electrode.
Any gases produced can be collected in the test tubes and tested (squeaky pop test - hydrogen, relight a glowing splint - oxygen).
hydrogen gas will form at the negative electrode
oxygen gas will form at the positive electrode
Level 3 (5-6 marks)
Wearing safety glasses, add sodium sulfate solution to a beaker, and connect two inert electrodes (make sure they do not touch) to a power supply.
Completely fill two small test tubes with sodium sulfate solution and position a test tube over each
electrode, then turn on the power supply and observe what happens at each electrode.
Any gases produced can be collected in the test tubes and tested (squeaky pop test - hydrogen, relight a glowing splint - oxygen).
hydrogen gas will
form at the negative electrode: 2H+(aq) + 2e- → H2(g)
oxygen gas will form at the positive electrode: 4OH-(aq) → 2H2O(l) + O2(g) + 4e-








