Aims of Experiment
Foundation Tier
Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration.
In this experiment you will:
- use a burette and colour change indicator to find out the volume of sulfuric acid that neutralises 25 ml of sodium hydroxide solution
Higher Tier
Determination of the reacting volumes of solutions of a strong acid and a strong alkali by titration.
Determination of the concentration of one of the solutions in mol/dm3 and g/dm3 from the reacting volumes and the known concentrations of other solutions
In this experiment you will:
- use a burette and colour change indicator to find out the volume of sulfuric acid that neutralises 25 ml of 0.1 mol/dm3 sodium hydroxide solution
- use your results to calculate the concentration of the sulfuric acid in mol/dm3 and in g/dm3
Risk Asessment
As a general rule, eye protection (goggles) must be worn for all practicals.
| hazard | possible harm | precaution |
|---|---|---|
| dilute sodium hydroxide solution |
skin irritation and serious eye irritation |
wear gloves, use a pipette filler |
| dilute hydrochloric acid |
skin irritation and eye irritation |
fill burette slowly (below eye level), using a funnel |
This risk assessment is provided as an example only, and you must perform your own risk assessment before doing this experiment.
Apparatus
Each group will need:
burette
burette stand or retort stand with burette clamp
plastic funnel to fit in top of burette
25.0 ml pipette
pipette filler
conical flask
white tile
phenolphthalein indicator
hydrochloric acid
sodium hydroxide solution
access to distilled/de‐ionised water
Experiment Set-up
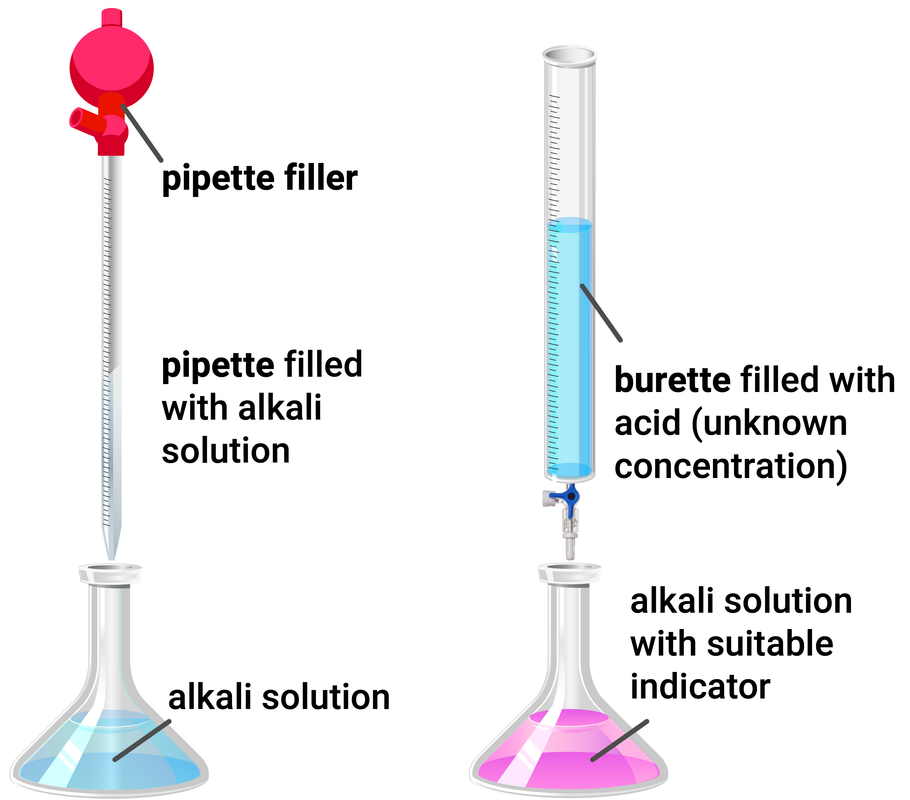
Method
- use a pipette and pipette filler to add 25 ml of sodium hydroxide to a clean conical flask
- add a few drops of phenolphthalein indicator and place the conical flask on a white tile
- fill the burette with hydrochloric acid and record the starting volume
- slowly open the tap of the burette, and add the acid to the conical flask, swirling to mix
- stop adding the acid when the end-point is reached (when the colour permanently changes from pink to colourless) and record your final volume
- repeat steps 1-5 until you get concordant titres (results are within 0.10 ml of each other)
Results and Analysis
| rough | run 1 | run 2 | run 3 | |
|---|---|---|---|---|
| final volume (ml) | 26. 55 | 26.35 | 26.80 | 26.25 |
| starting volume (ml) | 0.00 | 0.15 | 0.50 | 0.00 |
| titre (ml) | 26.55 | 26.20 | 26.30 | 26.25 |
Readings should be recorded to two decimal places, ending in 0 or 5.
The titre is the volume added (the difference between the final and starting volumes).
Select at least two concordant titres (these are titres within 0.10 ml of each other) and work out an average titre.
Use this volume of acid to work out its concentration.
Exam Question and Model Answer
A student has to check if two samples of hydrochloric acid, A and B, are the same concentration.
Describe how the student could use the apparatus (burette, pipette, conical flask, white tile) and the solutions (indicator, HCl A, HCl B, NaOH solution) to carry out titrations.
[6 marks]
Level 1 (1-2 marks)
Use a pipette to measure the acid into a conical flask, then add alkali from the burette.
Level 2 (3-4 marks)
Add hydrochloric acid, using a pipette, into a conical flask.
Fill a burette with the sodium hydroxide solution, then add a few drops of indicator into the conical flask.
Slowly add the alkali to the flask and swirl the flask whilst looking for a colour change.
Once a sudden colour change has occurred, stop adding alkali, and record the volume of alkali added from the burette.
Level 3 (5-6 marks)
Ensure you are wearing safety goggles and measure 25 ml of hydrochloric acid A, using a pipette and pipette filler, into a conical flask.
Fill a burette safely with the sodium hydroxide solution, then add a few drops of indicator into the conical flask.
Slowly add the alkali, drop by drop, to the conical flask and swirl the flask whilst looking for a colour change. Using a white tile under the flask can help to see this.
Once a sudden colour change has occurred, stop adding alkali, and record the volume of alkali added from the burette.
Repeat this again for the hydrochloric acid B - and if the same volume of alkali neutralises the acids, the two acids are of the same concentration.








