Aims of Experiment
Activity 1
How does the concentration of an acid affect the rate of reaction?
In this experiment you will:
- react magnesium ribbon with different concentrations of hydrochloric acid
- measure the volume of gas produced for each concentration
- use your results to work out how the rate of reaction is affected by the concentration of the acid
Activity 2
How does the concentration of sodium thiosulphate affect the rate of reaction?
In this experiment you will:
- react different concentrations of sodium thiosulfate with hydrochloric acid
- use a stop clock to time how long it takes for the mixture to become cloudy for each concentration
- use your results to work out how the rate of reaction changes as the concentration of the sodium thiosulfate changes
Risk Asessment
As a general rule, eye protection (goggles) must be worn for all practicals.
| hazard | possible harm | precaution |
|---|---|---|
| hydrochloric acid |
skin and eye irritation |
avoid contact with the skin |
| gases escaping from reaction |
may damage skin and eyes |
place cotton wool at opening of conical flask to minimse gas escape |
| hot sodium thiosulfate solution |
burns to the skin |
do not heat above 60°C |
| sulfur dioxide |
irritation to the eyes and lungs, particularly to people with asthma |
lab needs to be well ventilated |
This risk assessment is provided as an example only, and you must perform your own risk assessment before doing this experiment.
Apparatus
Each group will need:
magnesium strips
hydrochloric acid (3 concentrations)
250 ml conical flask
100 ml gas syringe
water bath
sodium thiosulfate
50 ml measuring cylinder
stop clock or stopwatch
10 ml measuring cylinder
Experiment Set-up
Activity 1
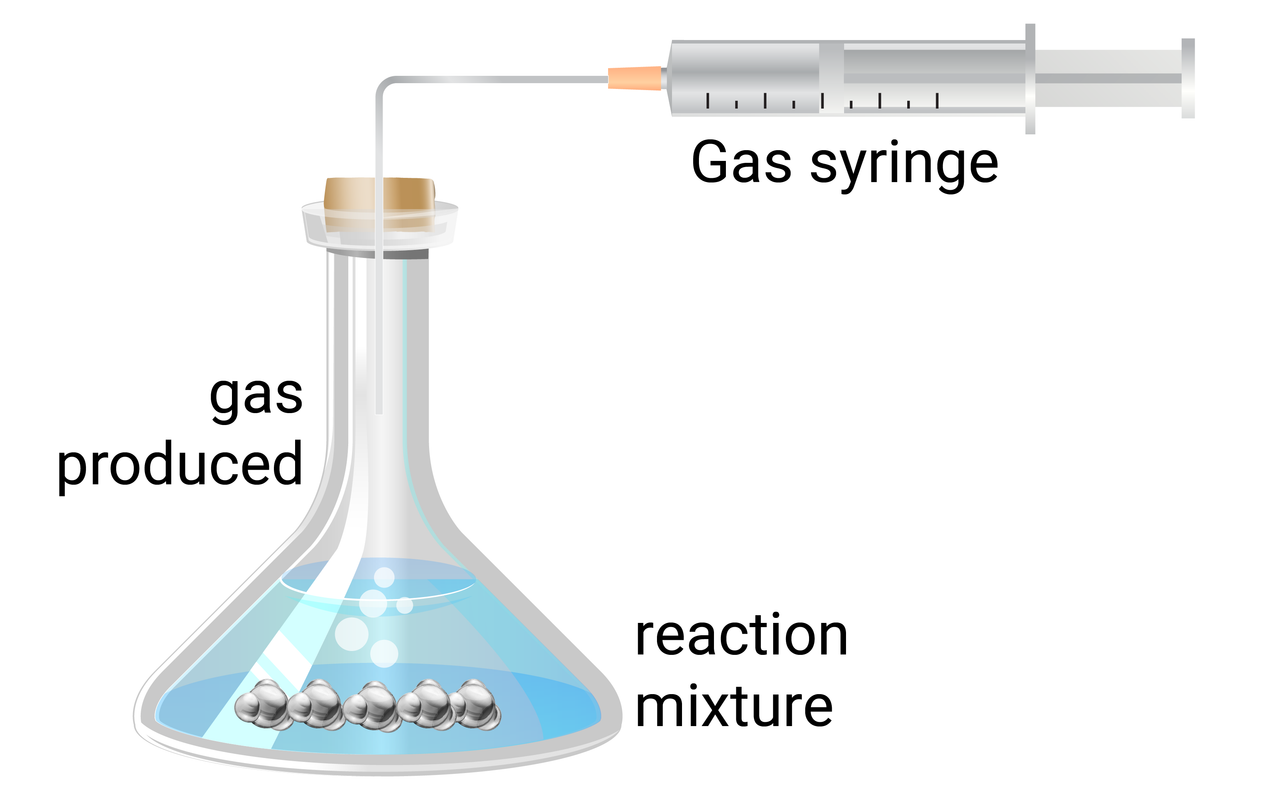
Activity 2
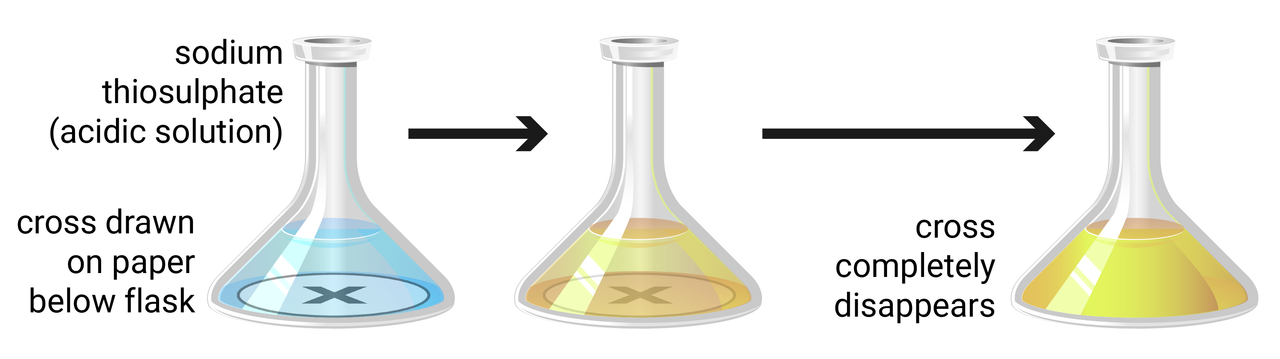
Method
Activity 1
- use a measuring cylinder to add 50 ml of 0.5 mol/dm3 hydrochloric acid to a conical flask
- add a single 3 cm strip of magnesium ribbon to the flask, and immediately connect the gas syringe and start a timer
- at every 20 seconds, record how much gas has been produced
- when the reaction is complete, clean the apparatus as instructed by your teacher
- repeat steps 1-4 with different concentrations (1.0 mol/dm3, and 1.5 mol/dm3) of hydrochloric acid
Activity 2
- use a measuring cylinder to add 10 ml of sodium thiosulfate solution to a conical flask, then add 40 ml of water (concentration 8 g/dm3)
- measure and record the temperature of the solution
- place the conical flask on a piece of paper with a black cross drawn on it
- use another measuring cylinder to add 10 ml of hydrochloric acid to the flask, and immediately start a timer
- when the cross is no longer visible record the time taken, and then clean the apparatus as instructed by your teacher
- repeat steps 1-5 changing the concentration of sodium thiosulphate each time as below:
- 20 ml sodium thiosulfate + 30 ml water (concentration 16 g/dm3)
- 30 ml sodium thiosulfate + 20 ml water (concentration 24 g/dm3)
- 40 ml sodium thiosulfate + 10 ml water (concentration 32 g/dm3)
- 50 ml sodium thiosulfate + no water (concentration 40 g/dm3).
- 20 ml sodium thiosulfate + 30 ml water (concentration 16 g/dm3)
Results and Analysis
Activity 1
| time (s) | volume of gas produced (ml) | ||
|---|---|---|---|
| 0.5 mol/dm3 | 1.0 mol/dm3 | 1.5 mol/dm3 | |
| 0 | 0 | 0 | 0 |
| 20 | |||
| ... | |||
For each concentration plot a graph on the same set of axes to show:
- volume of gas (ml) on the Y axis (vertical)
- time (s) on the X axis (horizontal)
- a curve of best fit
Use your graph to compare the rates of reaction with different concentrations of hydrochloric acid with magnesium.
Use collision theory to explain your findings.
Activity 2
| concentration (g/dm3) | time for cross to disappear (s) | ||
|---|---|---|---|
| trial 1 | trial 2 | mean | |
| 8 | |||
| 16 | |||
| ... | |||
Plot a graph to show:
- mean time (s) on the Y axis (vertical)
- concentration (g/dm3) on the X axis (horizontal)
- a curve of best fit
Describe the relationship between the independent variable and the dependent variable? What were your control variables?
Evaluate the two methods that you have used to investigate the effect of concentration on rate of reaction.
Exam Question and Model Answer
A chemical company makes calcium chloride by reacting calcium carbonate and hydrochloric acid. They think they can increase the rate of reaction by increasing the concentration of the acid. Describe an experiment they could do in a laboratory to be able to test this idea.
[6 marks]
Level 1 (1-2 marks)
Add calcium carbonate powder to a conical flask.
Pour hydrochloric acid into the flask, mix, and immediately attach a gas syringe.
Measure how much gas has been produced every 20 seconds, and record in a table.
Compare the results to find out which reaction has a faster rate.
Level 2 (3-4 marks)
Add calcium carbonate powder to a conical flask.
Pour hydrochloric acid into the flask, mix, and immediately attach a gas syringe.
Measure how much gas has been produced every 20 seconds, and record in a table.
Repeat these steps with other concentrations of hydrochloric acid as well (keep the volume the same).
Compare the results to find out which reaction has a faster rate.
Level 3 (5-6 marks)
Add 5 g of calcium carbonate powder to a conical flask.
Using a measuring cylinder, pour and mix 20 ml of 0.5 mol/dm3 hydrochloric acid into the flask, and immediately attach a gas syringe.
Measure how much gas has been produced every 20 seconds, and record in a table.
Repeat these steps with 1.0 mol/dm3 and 1.5 mol/dm3 hydrochloric acid as well (keeping the temperature, volume of acid the same, and the mass of the calcium carbonate the same).
Calculate the rate of each reaction (volume ÷ time), and compare the results to find out which reaction has a faster rate.








